Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: A hallmark of rheumatoid arthritis (RA) is the presence of anti-citrullinated protein antibodies (ACPA) targeting neoantigens which are generated by a family of enzymes called protein-arginine deiminases (PAD). Among the five members of the PAD family (PAD1, PAD2, PAD3, PAD4 and PAD6), PAD4 has been reported to play a key role in RA pathogenesis. Besides ACPA, about 25-35% of RA patients express anti-PAD4 IgG of which about 20-40% cross-react between PAD3/PAD4 and have the potential to enhance PAD4 activity leading to more erosive disease. Although several studies have been published on cross-sectional RA cohorts and controls, limited data is available from controlled clinical trials. Consequently, the aim of this study was to evaluate the prevalence of anti-PAD2, anti-PAD3 and anti-PAD4 IgG and IgA in a cohort of established RA patients, and the impact of treatment on antibody (Ab) levels, in the adalimumab vs. abatacept head-to-head active RA AMPLE study (NCT00929864).
Methods: Sera from RA patients enrolled in the AMPLE trial [at baseline (n=498), and 1 year after treatment initiation (n=218)] and from age- and sex-matched healthy controls (n=129) were tested for ACPA IgG (ImmunoscanCCPlus, Svar, Malmö, Sweden), anti-PAD2, anti-PAD3 and anti-PAD4 IgG/IgA by a novel particle-based multi-analyte technology [PMAT, research use only (RUO), Werfen, San Diego, US]. The manufacturer cut-off was used for ACPA, and preliminary cut-offs determined with internal disease and healthy controls were used for the anti-PAD Abs. Joint erosion (JE) data as measured by van der Heijde modified Total Sharp Score were compared with the Ab levels by Spearman’s correlation. Anti-PAD4 IgG results were normalized to total IgG levels for analysis of changes with treatment by t-test.
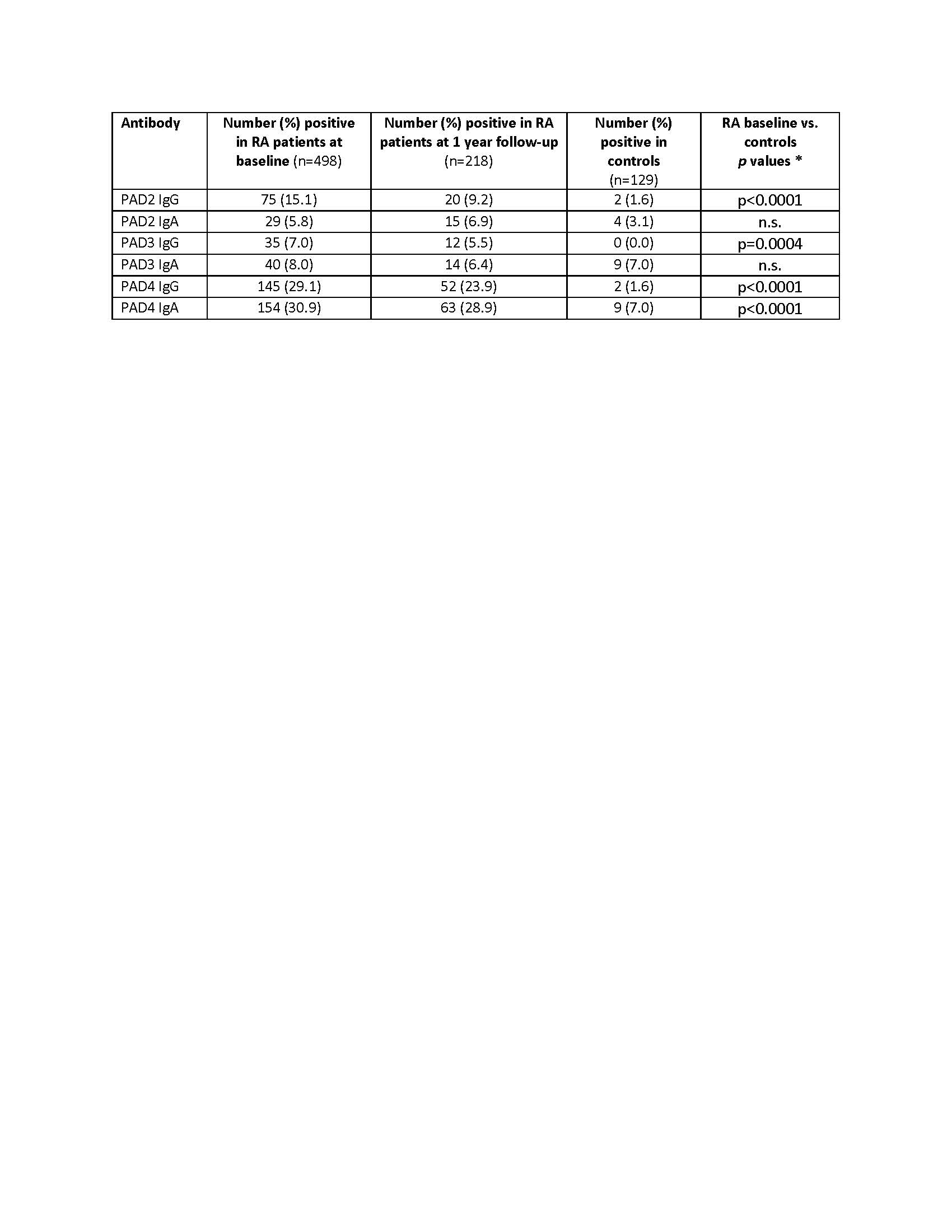
Results: Profiling of Abs to PAD2, PAD3 and PAD4 using the preliminary cut-offs identified both IgG and IgA in RA patients (Table 1). Except for anti-PAD2 and anti-PAD3 IgA, all other PAD autoantibodies were more prevalent in RA patients vs. the matched controls. At baseline, the highest prevalence of Abs was found for PAD4 IgA followed by PAD4 IgG, PAD2 IgG, PAD3 IgA, PAD3 IgG and PAD2 IgA. Twenty nine percent (145/498) of RA patients were anti-PAD4 IgG+ vs. 2/129 (1.6%) of controls (p < 0.0001). The presence of anti-PAD4 IgG was significantly higher in ACPA+ RA patients [139/387 (35.9%)] compared to the ACPA- group [6/117 (5.1%)] (p < 0.001, Fisher exact test). A weak correlation was observed when comparing anti-PAD4 IgG titers with JE and disease duration (ρ= 0.13 and 0.18, p=0.0045 and 5.1 x 10-5, respectively). Positivity for anti-PAD2, anti-PAD3 or anti-PAD4 IgG or IgA did not predict response to either adalimumab or abatacept. Anti-PAD4 IgG levels were generally stable at 1 year of both treatments, with a slight observed reduction in the adalimumab group when normalized to total IgG.
Conclusion: In the head-to-head adalimumab vs abatacept trial, anti-PAD4 IgG were weakly associated with JE and longer disease duration in active RA patients, which is consistent with previous reports. Anti-PAD IgG or IgA status did not predict response to treatment, and treatments had little to no impact on titers after 1 year.
To cite this abstract in AMA style:
Kumar N, Chen X, Aure M, Ishii A, Martinez-Prat L, Bentow c, Saini J, Mahler M, Menard L. Profiling of Anti-PAD IgG and IgA in Patients from the Head-to-head Adalimumab vs Abatacept Rheumatoid Arthritis AMPLE Trial and Matched Healthy Controls [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/profiling-of-anti-pad-igg-and-iga-in-patients-from-the-head-to-head-adalimumab-vs-abatacept-rheumatoid-arthritis-ample-trial-and-matched-healthy-controls/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/profiling-of-anti-pad-igg-and-iga-in-patients-from-the-head-to-head-adalimumab-vs-abatacept-rheumatoid-arthritis-ample-trial-and-matched-healthy-controls/