Session Information
Date: Monday, November 11, 2019
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: An M2-like alternative activation state of macrophages has been linked to the pathogenesis of several fibrotic disorders, including systemic sclerosis (SSc). MRTF-A is an essential mechanosensing transcription factor involved in cellular responses to stiff fibrotic tissue, but its role in controlling macrophage polarisation is unknown. However, genome database profiling identifies the promoter region of the interleukin 13 receptor gene, IL13Rα1, critical to M2 polarisation, as a target of MRTF-A.
Methods: MRTF-A knockout (KO) and wild type (WT) control mice, were subject to excisional wound healing and ex vivo polarisation of bone marrow-derived macrophages, in order to model M2-responses. Peripheral blood mononuclear cells from SSc patients (N=9) were cultured in M-CSF (4ng/ml) for 7 days to derive M2-like human macrophages. 50kPa Softwell gels were used to reproduce the effect of stiff tissue microenvironments. Morphology (elongated M2-like, round M1-like), and gene expression by qPCR were used to profile the macrophage activation state (IFNγ for M1-like, Arg1 and CD206 M2-like, IL13Rα1 predicted MRTF-A responsive, and IL-4R, predicted non-responsive). Secreted TGFb was assayed by ELISA. CCG-257081 (CCG) (10µM), an MRTF-A pathway inhibitor, was used to block MRTF-A.
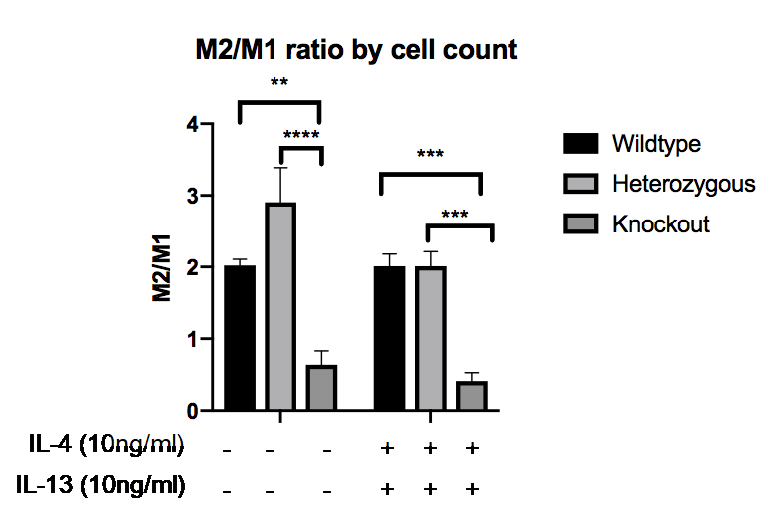
Results: In mice, MRTF-A WT (2.02±0.086, mean±SEM) and MRTF-A Het macrophages (2.24±0.716) exhibited significantly higher M2/M1 cell morphology ratio compared to MRTF-A KO macrophages (0.640±0.193) (p=0.0373 and p=0.0165 respectively)(see figure below). Excisional wound repair was also delayed (day 7, WT 47.7±13.9, KO 98.6±19.3, p< 0.037; day 11, WT 3.3 ±1.1, KO 27.4 ± 4.1 p< 0.011, % basal wound area). SSc macrophages showed M2-like activation signature under basal conditions (high Arg1, CD206, M2-morphology), partially reversed by CCG, reducing CD206 (basal 9.16 ± 4.26, CCG treated 1.79±0.98, p=0.0039), supressing the elongated M2-like morphology (SSc basal 4.04, ±0.74, cells per field, CCG treated 0.46±0.18, P=0.0017), and inhibiting the secretion of TGFβ (SSc basal 7.0± 4.9 pg/ml, CCG treated 0.0±1.7 P=0.039). The M1-like inflammatory marker IFNγ was increased by CCG treatment consistent with repolarisation (SSc basal 2.02±0.54, CCG treated 10.4±3.6, p=0.0039). As a direct target of MRTF-A, IL13Rα, was decreased by CCG (21.5±3.39) compared to control (83.2±19.9, p=0.0252), and in mice KO macrophages showed decreased IL13Rα1:IL4R ratio, indicating a pathway selective effect. However, paradoxical elevation of Arg1 in some SSc cells following CCG treatment (SSc basal 7.11±2.89, CCG treated 19.19±8.31, p=0.027), and induction of Arg1 in KO mouse macrophages by polarising cytokines, indicates that not all M2-like responses were affected.
Conclusion: Additional to a pivotal role in myofibroblast function, loss of MRTF-A results in reduced IL-13Rα1 and attenuated cytoskeletal changes associated with M2-like polarisation. Experiments using the CCG-257081 inhibitor in human macrophages support this model, and indicate possible therapeutic potential in inhibiting the MRTF-A mechanosensing pathway by targeting IL4/13.
To cite this abstract in AMA style:
Lim T, Krogmannova K, Xu S, Ahmed Abdi B, Abraham D, Denton C, Stratton R. Profibrotic Macrophage Activation in Systemic Sclerosis Is Dependent on the Mechanosensing MRTF-A Pathway [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/profibrotic-macrophage-activation-in-systemic-sclerosis-is-dependent-on-the-mechanosensing-mrtf-a-pathway/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/profibrotic-macrophage-activation-in-systemic-sclerosis-is-dependent-on-the-mechanosensing-mrtf-a-pathway/