Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) is the most common chronic inflammatory rheumatic disease, characterized by synovitis associated with progressive bone loss and joint degradation. The adjuvant-induced arthritis (AIA) model mimics the pathophysiological features of RA, with high prevalence and reproducibility. Yes-associated protein (YAP) is a transcription factor involved in inflammatory signaling and arthritis progression, especially by driving the RA fibroblast-like synoviocyte (FLS) phenotype. Beyond inflammation sensing, YAP responds to mechanical stimuli. The inhibition of YAP transcriptional activity decreases arthritis in the AIA model. The objective of this study was then to investigate the effect of mechanical loading or unloading on arthritis.
Methods: For in vitro studies, a compressive dynamic pressure (100mmHg) was applied on RA FLS organoids. For in vivo studies, mechanical loading (voluntary running), unloading (tail suspension) or no mechanical stress (control) was applied in AIA rats (n=11/group), from arthritis induction (day[D]0) until D17. Running rats only ran (1.5km/day) from D0 to D10, which is the arthritis onset in the AIA model. Daily clinical monitoring was assessed to follow the severity of arthritis. In vivo µ-computed tomography was realized to follow arthritis-induced bone loss.At D10 or D17, the ankles of the rats were collected to perform RT-qPCR. At D17, blood samples were collected to perform protein dosages.
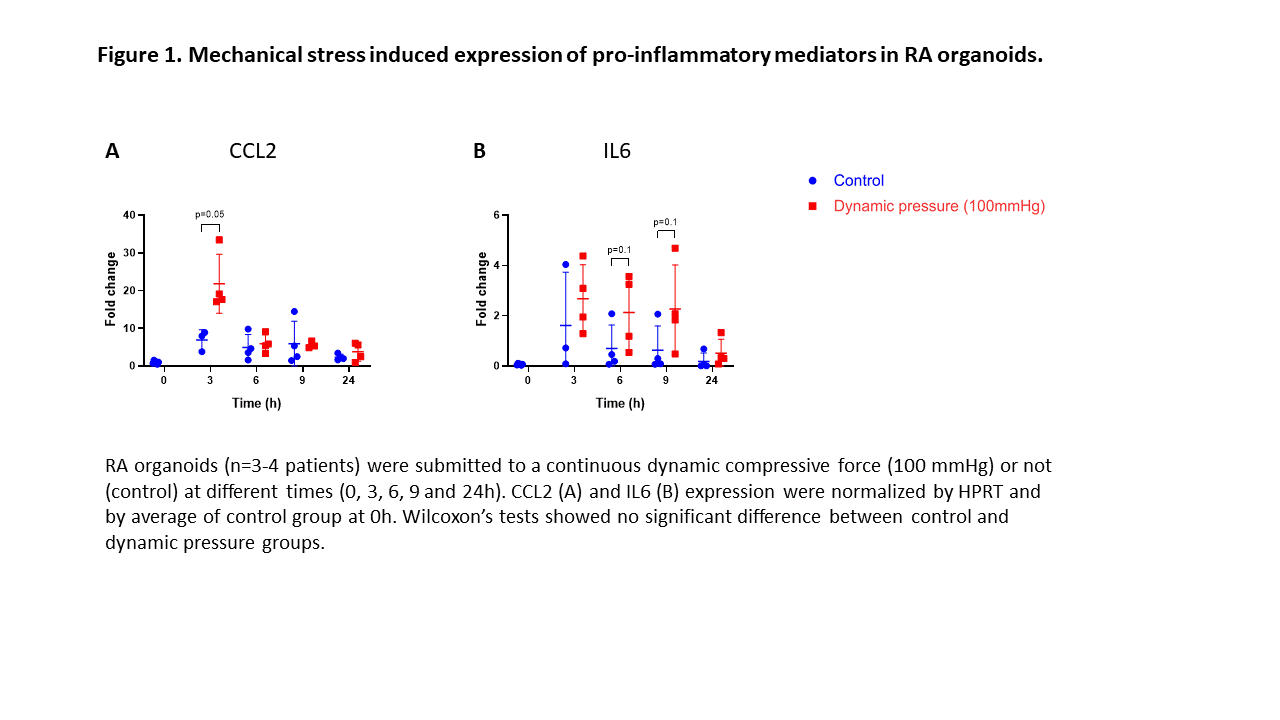
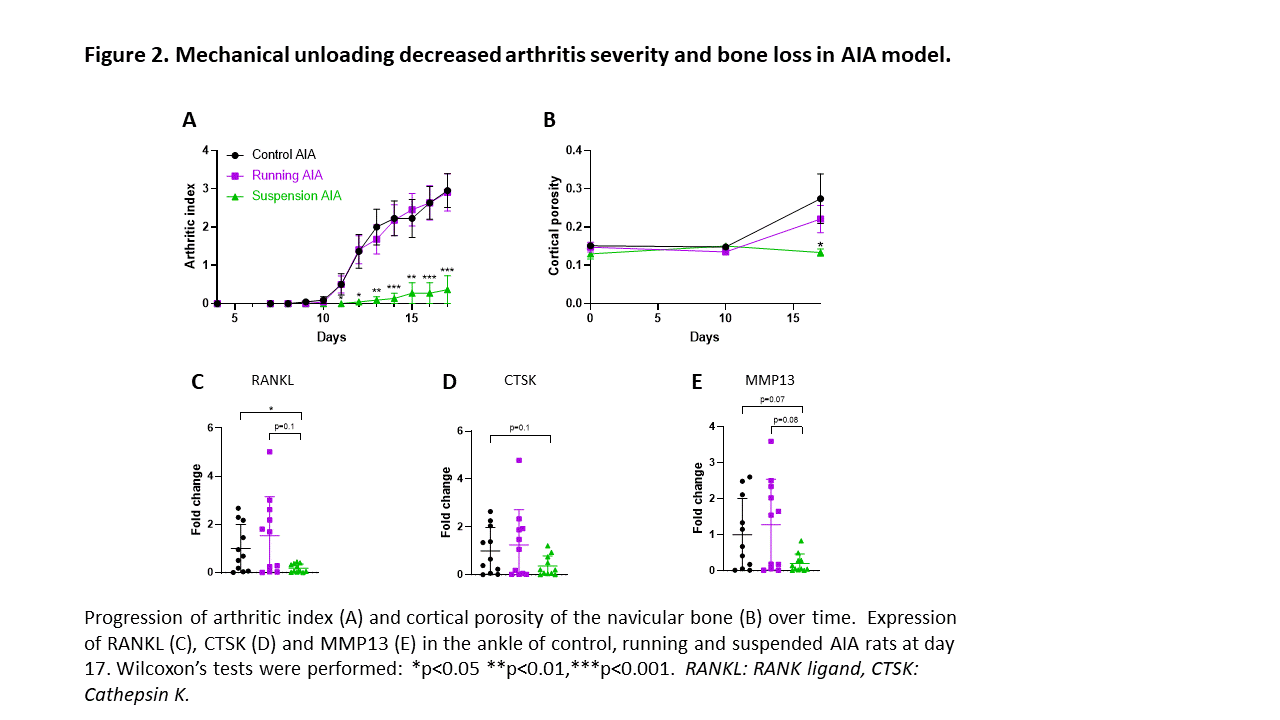
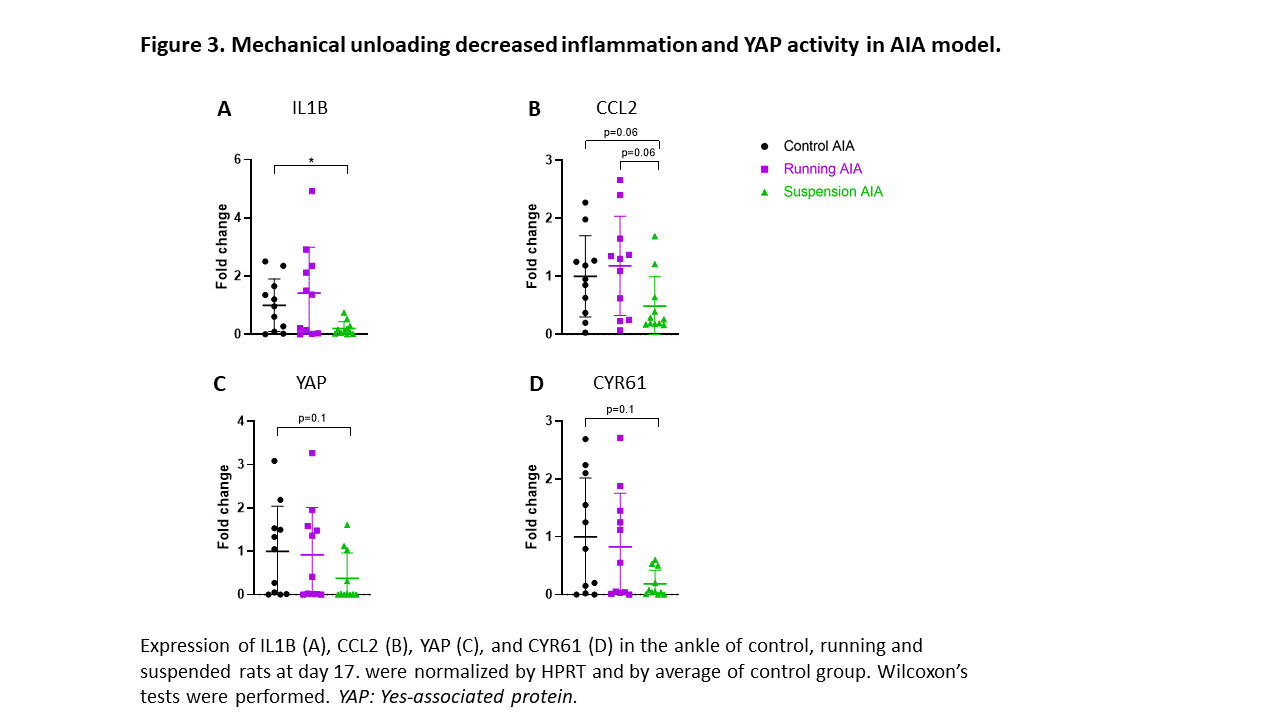
Results: In RA organoids, mechanical stress was able to induce inflammation by increasing IL6 and CCL2 expression (Figure 1). Surprisingly, YAP transcriptional activity remained unchanged in response to mechanical stress in RA organoids. In AIA rats, CCL2 expression is increased before arthritis onset, but also during and after the peak of inflammation (D17), independently from YAP activity. In AIA rats, the control and running groups displayed the same disease onset (D10) and progression, with similar arthritis index and ankle circumference over time (Figure 2). No difference in expression of bone degradation markers (RANK ligand, Cathepsin K, MMP9, and MMP13) was observed in joint at D17 between the control and running groups (Figure 2) and pro-inflammatory mediators (IL6, IL1B, TNF, and CCL2) (Figure 3). However, the expression of pro-inflammatory mediators and bone degradation markers trended to increase in the running AIA rats joint at D10. The suspension prevented arthritis development with a decreased expression of bone degradation markers (Figure 2) and pro-inflammatory mediators in the joint at D17 (Figure 3). Moreover, arthritis-induced bone loss was inhibited in the suspension group, where cortical porosity and trabecular volume remained similar to baseline.YAP transcriptional activity also trended to decrease.
Conclusion: In conclusion, two independent mechanisms could be involved in arthritis in response to mechanical stress, one mediated by YAP and the other involving CCL2. Mechanical unloading might then prevent arthritis by decreasing YAP activity and CCL2 production. A high level of CCL2 might then increase arthritis severity by inducing inflammation at arthritis onset, leading to an increase in YAP activity and joint degradation.
To cite this abstract in AMA style:
Dalix E, Maalouf M, Caire R, Courbon G, Dumas V, Peyroche S, Linossier M, Thomas M, Arthaud C, Vanden-Bossche A, Marotte H. Prevention of Arthritis Development by Mechanical Unloading Through Inhibition of CCL2 and YAP-mediated Inflammation in the Rat Adjuvant-induced Arthritis Model [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/prevention-of-arthritis-development-by-mechanical-unloading-through-inhibition-of-ccl2-and-yap-mediated-inflammation-in-the-rat-adjuvant-induced-arthritis-model/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevention-of-arthritis-development-by-mechanical-unloading-through-inhibition-of-ccl2-and-yap-mediated-inflammation-in-the-rat-adjuvant-induced-arthritis-model/