Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Biologics have been advocated by guidelines as effective therapies for rheumatoid arthritis (RA)1. Limited longitudinal studies investigated the prevalence and risk factors for developing adverse drug reactions (ADRs) in RA patients treated with intravenous (IV) originator biologics2. Moreover, none of these studies utilized Naranjo3 probability assessment for ADRs and compared all IV biologics in one setting.
Methods: A retrospective cohort study of adult RA patients receiving IV abatacept, tocilizumab, infliximab, or rituximab from January 2015 to January 2020 in a tertiary hospital located in Riyadh, Saudi Arabia was conducted. After Naranjo probability assessment 1of ADRs, data including demographics, comorbidities, and concomitant medications were retrieved and their contribution to ADRs was analysed by logistic regression. Time to develop ADR for each biologic was plotted using Kaplan Meier graph.
Results: A total of 129 eligible patients were included in the study taking tocilizumab, rituximab, abatacept and infliximab (38.76%, 38.76%,13.95% and 8.53%, respectively) with a total of 1963 doses administered over five years. Patients had a mean (±SD) age of 54 (±13) years and were mostly females (87.6%). In addition to biologics used, 72 patients (55.8%) were receiving methotrexate, and 29 (22.5%) were receiving other disease modifying anti-rheumatic drugs (DMARDs). A total of 282 ADRs with Naranjo score≥1 was experienced by 103 patients (79.84%). The most common ADRs were infections (26.6%), skin and mucous membrane disorders (14.18%), and gastrointestinal disorders (11.7%) with predominance of probable ADRs using Naranjo classification (Table 1). Unadjusted odds ratio (OR) and adjusted odds ratio (AOR) for biologics and other factors are available in table 2. Experiencing multiple ADRs was significant with abatacept users with an AOR of 3.15 [95% CI: 1.004-9.854, p= 0.049]. The time to experience ADRs was ranging from day zero to three years (Figure 1).
Conclusion: Intravenous biologics use is limited by the prevalence of ADRs globally. Knowledge of different types of ADRs and their expected occurrence aids with clinical decision making and provides a database for future comparison with biosimilars. Although this study had a small number of participants for some IV biologics, the longitudinal nature of study methods provides an insight on the safety profile while managing RA patients.
References
- Gartlehner G. et al. J Rheumatol. 2006, 33(12):2398-408.
- Rein P. et al. Rheumatology and Therapy 2017, 4(2):247–261.
- Naranjo C. et al. Clinical pharmacology and therapeutics 1981, 30(2):239-245.
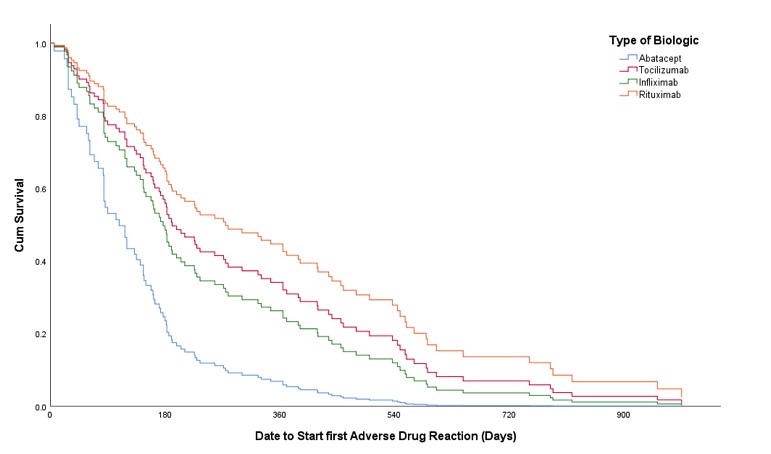 Figure 1. Cox regression and resulting Kaplan Meier graph of different IV originator biologics that shows prediction of having at least one ADR with Naranjo scale ≥1 and its relation to time (days) of experiencing the first ADR
Figure 1. Cox regression and resulting Kaplan Meier graph of different IV originator biologics that shows prediction of having at least one ADR with Naranjo scale ≥1 and its relation to time (days) of experiencing the first ADR
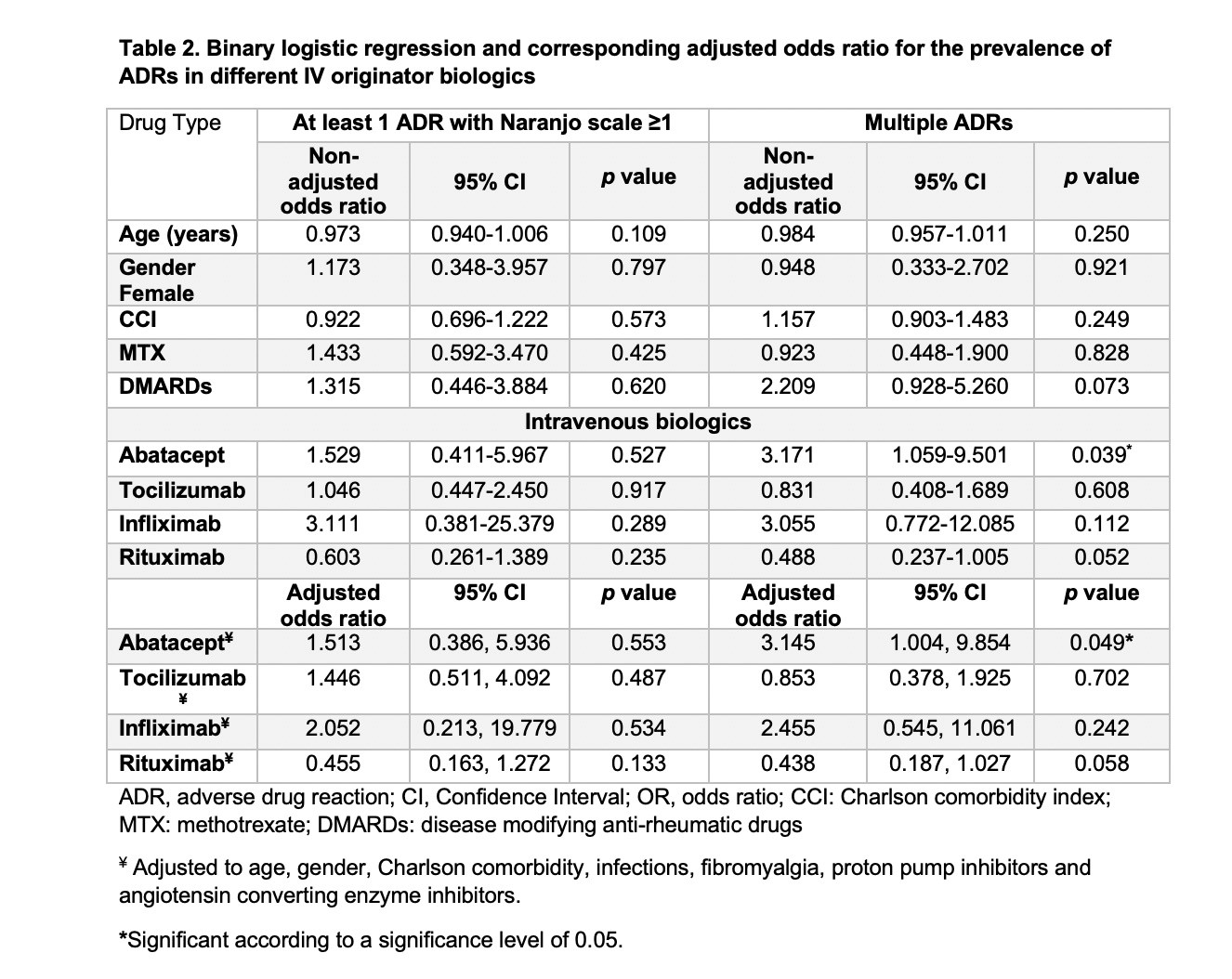 Table 2. Binary logistic regression and corresponding adjusted odds ratio for the prevalence of ADRs in different IV originator biologics
Table 2. Binary logistic regression and corresponding adjusted odds ratio for the prevalence of ADRs in different IV originator biologics
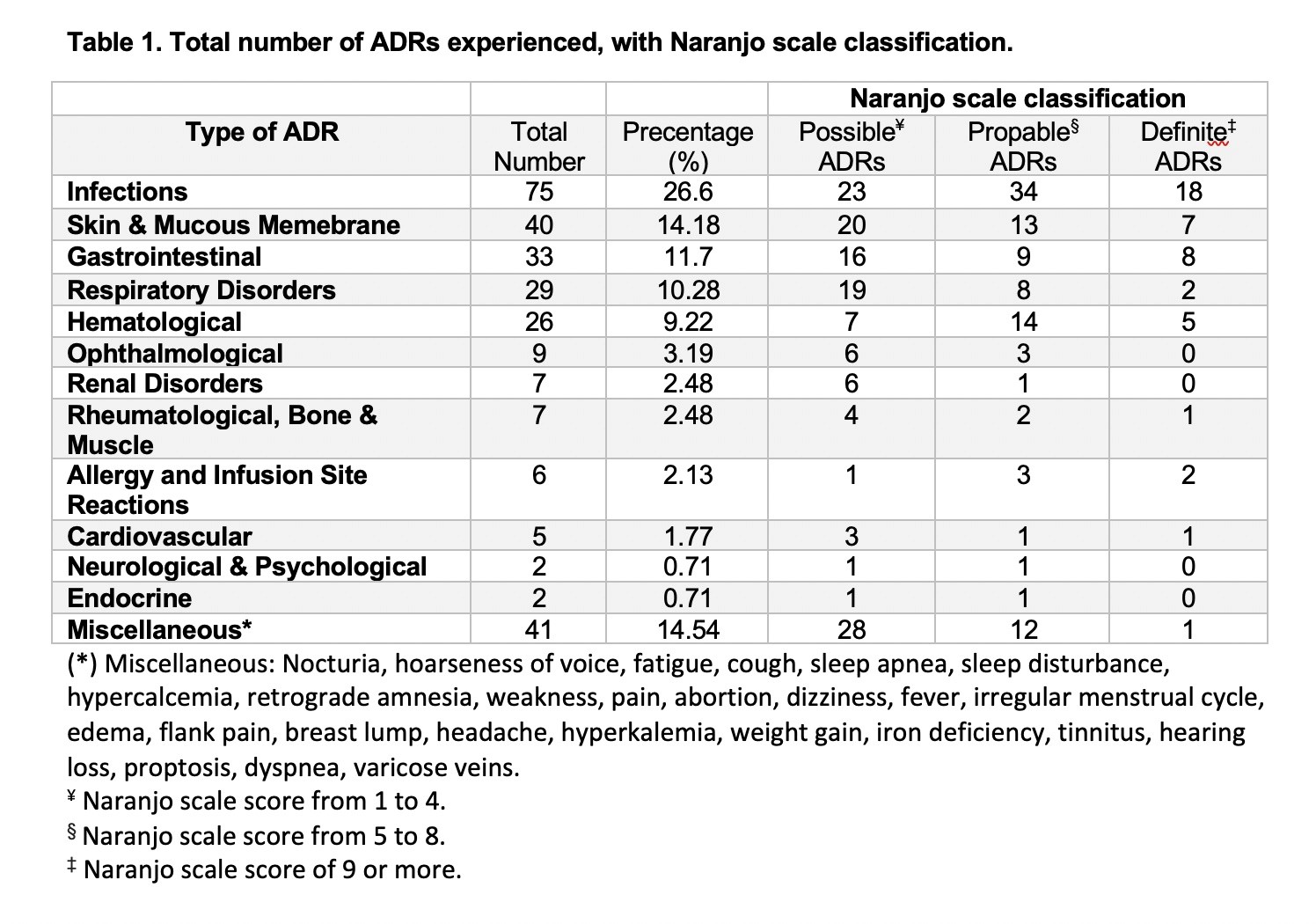 Table 1. Total number of ADRs experienced, with Naranjo scale classification.
Table 1. Total number of ADRs experienced, with Naranjo scale classification.
To cite this abstract in AMA style:
Alhazzani H, Tashkandi R, Alzamel L, Almalag H, Alaujan S, Alarfaj A, Alarfaj H, Omair M. Prevalence and Probability Assessment of Adverse Drug Reactions in Rheumatoid Arthritis Patients Receiving Intravenous Originator Biologics in Saudi Arabia: A Longitudinal Five Years Follow-up Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/prevalence-and-probability-assessment-of-adverse-drug-reactions-in-rheumatoid-arthritis-patients-receiving-intravenous-originator-biologics-in-saudi-arabia-a-longitudinal-five-years-follow-up-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevalence-and-probability-assessment-of-adverse-drug-reactions-in-rheumatoid-arthritis-patients-receiving-intravenous-originator-biologics-in-saudi-arabia-a-longitudinal-five-years-follow-up-study/
