Session Information
Date: Monday, November 9, 2020
Title: RA – Diagnosis, Manifestations, & Outcomes Poster IV: Lifespan of a Disease
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: RA patients have an increased risk of infection1 and venous thromboembolism (VTE)2. Although rates of serious infection and VTE have been reported for Medicare recipients with RA, the scope of previous publications has been limited to a select number of targeted immunomodulators3,4. This study examined the prevalence and incidence of infection and VTE in US Medicare RA patients initiating any of the available DMARDs.
Methods: Within the 20% sample of Medicare fee-for-service beneficiaries, the study population comprised of RA patients (without malignancy or non-RA autoimmune disease) with first initiation (index date; 01/2013–12/2015) of various classes of DMARDs: csDMARD, TNF-a inhibitor (TNFi), anti-IL6, anti-CD20, anti-CD80/86, and/or Janus kinase inhibitor (JAKi). The observation period involved baseline (one-year prior to index date) and follow-up (from index date until discontinuation, Medicare disenrollment, death, or 12/31/2016). Conditions of interest included VTE (deep-vein thrombosis and pulmonary embolism, defined based on diagnosis codes), overall infection (based on diagnostic codes or use of antibiotics/antivirals), serious infection (based on inpatient or emergency department diagnostic codes in any position, or outpatient use of intravenous antibiotics), opportunistic infection (based on diagnostic codes), and herpes zoster (HZ, based on diagnostic codes). We calculated baseline prevalence (%) and on-treatment incidence (number of new events per 100 patient-years [P100PY] during index DMARD treatment) of these conditions. Incident infection analyses excluded patients with infection within 60 days before index date. Incident VTE analyses excluded patients with any pre-index VTE.
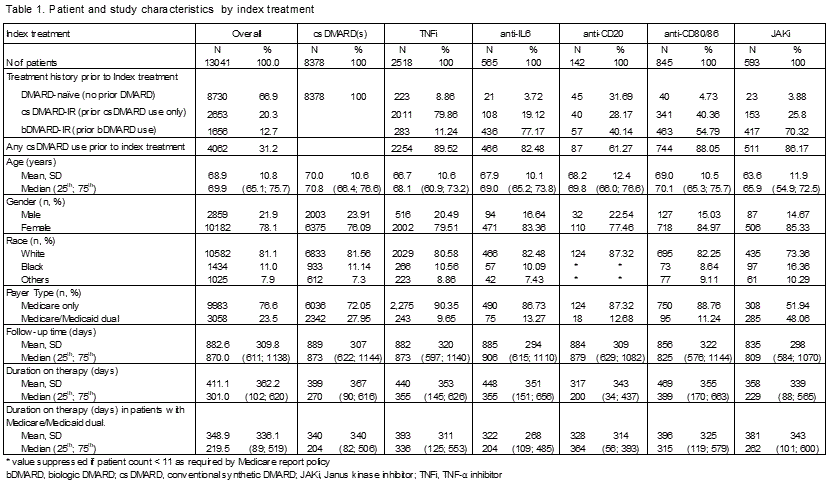
Results: The 13,041 RA patients (78.1% female; mean age 68.9 years at index date) initiated the following index therapies: csDMARD (64.3%), TNFi (19.3%), anti-IL6 (4.3%), anti-CD20 (1.1%), anti-CD80/86 (6.5%), and JAKi (4.5%). The median follow-up time ranged 2.2–2.5 years, and median time on index therapy ranged 0.5–1.1 years (Table 1).
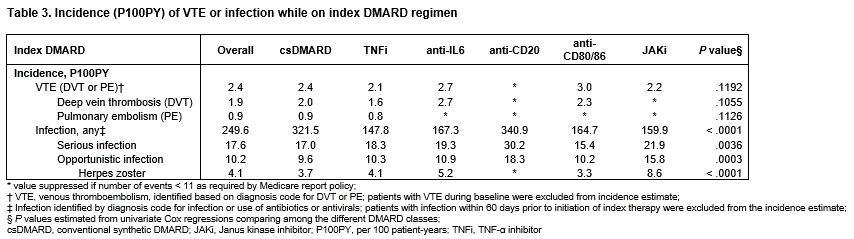
In the 12 months prior to initiating index therapy, patients experienced VTE (prevalence 4.4%) and infections (81.9%), serious infections (16.6%), opportunistic infections (9.7%), and HZ (3.4%). (Table 2). The on-therapy incidence P100PY was 2.4 for VTE (range 2.1–3.0), 249.6 for infection (range 147.8–340.9), 17.6 for serious infection (range 15.4–30.2), and 10.2 for opportunistic infection overall (range 9.6–18.3), including 4.1 for HZ (range 3.3–8.6) (Table 3).
Conclusion: VTE and infection affected Medicare beneficiaries with RA before initiating a new DMARD therapy. While on the new treatment regimen, patients developed recurrent or novel infections or novel VTE cases. Understanding the prevalence and incidence of these conditions can help clinicians and population-health decision makers optimize treatment choices.
References
- Doran MF, et al. Arthritis and rheumatism. 2002;46(9):2287–2293.
- Ungprasert P, et al. Clinical rheumatology. 2014;33(3):297–304.
- Pawar A, et al. Ann Rheum Dis 2019, 78:456–464.
- Desai RJ, et al. Arthritis Rheumatol 2019, 71(6):892–900.
To cite this abstract in AMA style:
Dore R, Antonova J, Chang L, Li S, Guo H, Ji Y, Genovese M. Prevalence and Incidence of Infection and Venous Thromboembolism in Rheumatoid Arthritis Patients Newly Initiating Various DMARD Classes: Real-World Analysis of 2012–2016 US Medicare Data [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/prevalence-and-incidence-of-infection-and-venous-thromboembolism-in-rheumatoid-arthritis-patients-newly-initiating-various-dmard-classes-real-world-analysis-of-2012-2016-us-medicare-data/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevalence-and-incidence-of-infection-and-venous-thromboembolism-in-rheumatoid-arthritis-patients-newly-initiating-various-dmard-classes-real-world-analysis-of-2012-2016-us-medicare-data/