Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Tocilizumab (TCZ) is an IL-6–receptor inhibitor for treatment of rheumatoid arthritis (RA) patients (pts). Because TCZ may impact how IL-6 modulates T-cell activation and regulates B-cell differentiation, response to vaccination in TCZ‑treated pts is of interest. VISARA is the first randomized controlled study of the effects of TCZ on response to 23-valent pneumococcal polysaccharide vaccine (23VPPV) and tetanus toxoid vaccine (TTV) in RA pts on stable MTX. Preliminary analysis of study endpoints and post hoc analyses assessing impact of concomitant oral corticosteroids (OCS) are presented.
Methods: RA pts with inadequate response/intolerance to ≥1 anti-TNF agent were stratified by age and randomly assigned (2:1) to TCZ 8 mg/kg IV every 4 wks + MTX (TCZ) or MTX only (MTX). Baseline (BL) serology samples were collected 3 wks after the first infusion, just before 23VPPV and TTV vaccination. Anti-pneumococcal and anti-tetanus antibody titers were evaluated at wk 8, after which all pts received TCZ+MTX for 12 more wks. Endpoints were proportion of pts with positive response (2-fold or >1 mg/L increase in serum antibody titers) to ≥6 of 12 23VPPV serotypes (primary) and proportion with positive response (4-fold or ≥0.2 mg/L increase in serum antibody titers) to TTV (secondary) at wk 8 (5 wks postvaccination).
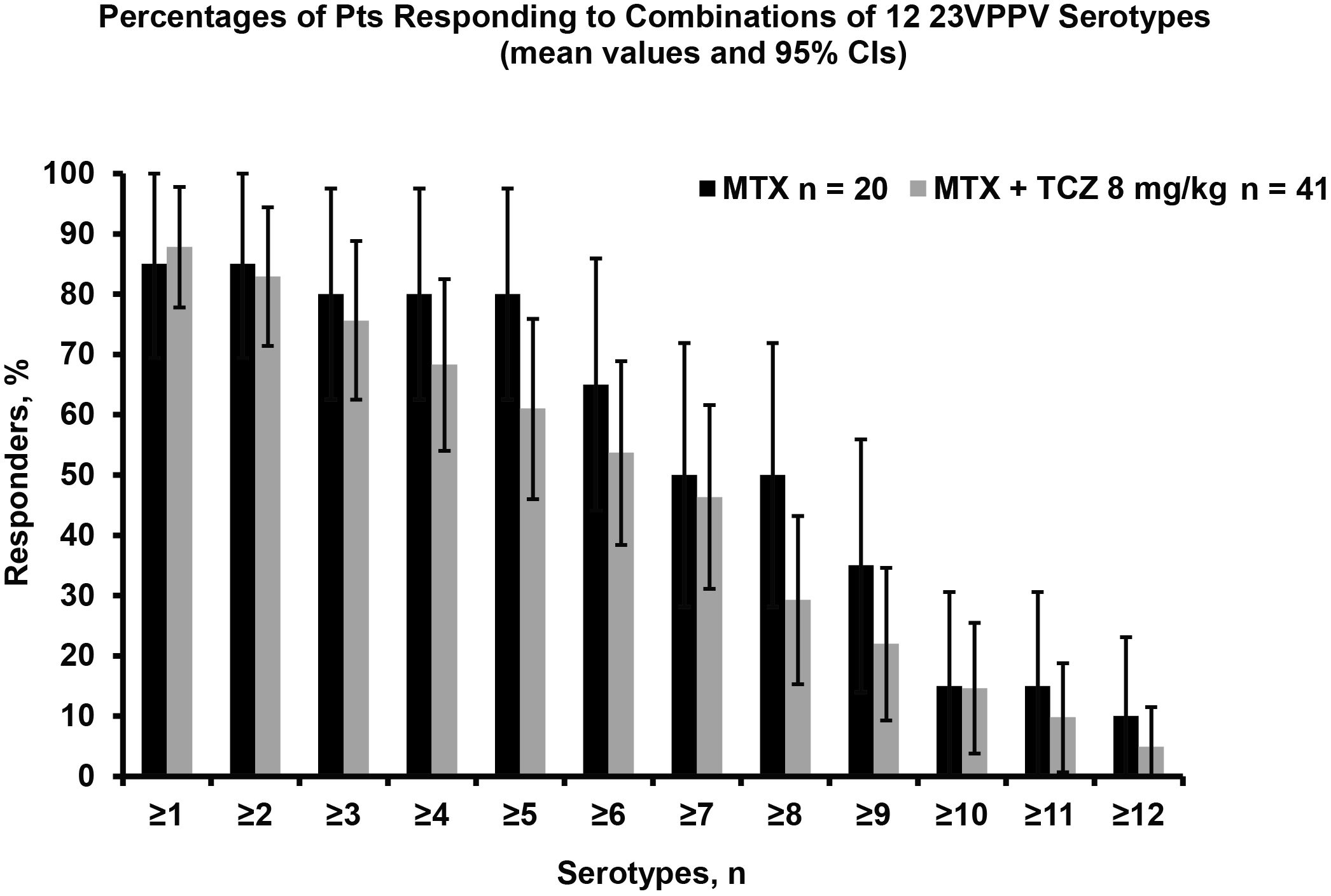
Results: Data are available for 74/91 enrolled pts and presented for 61 pts who met per-protocol criteria (ie, received ≥1 dose of study medication and both vaccines, had available BL and 8-wk titer samples and no protocol violations). At wk 8, 53.7% of TCZ+MTX and 65% of MTX pts responded to ≥6 of 12 23VPPV serotypes, a difference of 11.3% (not statistically significant at 95% CI level [CI: –37.3, 14.6]). When evaluated by age, the proportion of responders >50 y was lower by 10% in both arms. More MTX than TCZ+MTX pts responded to serotype combinations (from ≥1 to 12). However, the 95% CIs for the proportions of responders were wide and overlapping in both arms (Figure). A higher proportion of TCZ+MTX (43.9%) vs MTX pts (36.8%) responded to TTV, but the difference (7.1%) was not significant ([95% CI: –19.5%, 33.6%]). Two TCZ pts had nonprotective BL antibody titers (ie, <0.1 IU/mL); both achieved protective levels by wk 8. In pts taking OCS, the mean BL OCS dose was slightly higher in the responder than in the nonresponder MTX group, whereas the doses were similar in both TCZ+MTX groups (data not shown). Safety of TCZ was consistent with previous reports in RA pts.
Conclusion: Based on the 95% CI for the difference in proportions of responders, TCZ does not appear to significantly attenuate responsiveness to 23VPPV or TTV compared with MTX. However, given the small study sample size and the short duration of TCZ treatment before vaccination, in line with current recommendations, pts should, if possible, be up to date with required immunizations before initiating TCZ to maximize the vaccine response.
Disclosure:
C. O. Bingham III,
Genentech, Roche Pharmaceuticals,
5;
W. C. Rizzo,
Advanced Arthritis Care,
2,
UCB, Savient,
5,
Amgen, Takeda, UCB, Roche Pharmaceuticals. Abbott,
8;
M. Klearman,
Genentech and Biogen IDEC Inc.,
3;
A. Hassanali,
Genentech and Biogen IDEC Inc.,
3;
R. Upmanyu,
Roche Pharmaceuticals,
3,
Roche Pharmaceuticals,
1;
A. J. Kivitz,
Roche Pharmaceuticals,
8.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preliminary-results-from-a-controlled-study-assessing-the-humoral-immune-response-to-vaccines-in-rheumatoid-arthritis-patients-treated-with-tocilizumab/