Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose:
Rheumatologic disorders and inflammatory bowel disease can affect women of childbearing potential. Golimumab (GLM) is approved for rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis, polyarticular juvenile idiopathic arthritis and ulcerative colitis (UC). To characterize pregnancy outcomes in patients treated with GLM, data obtained from maternal exposure to GLM are presented.
Methods:
This dataset includes individual patient cases reported to the manufacturer through 06 April 2019. Cases included in the analysis were medically confirmed cases of maternal exposures to GLM during pregnancy or within 3 months prior to conception, and a reported pregnancy outcome. Both prospectively reported (ie, pregnancy outcome not known when first reported) and retrospectively reported cases (ie, pregnancy outcome known when first reported) were included. Cases originated from various sources, including spontaneous reporting, clinical studies, and registries.
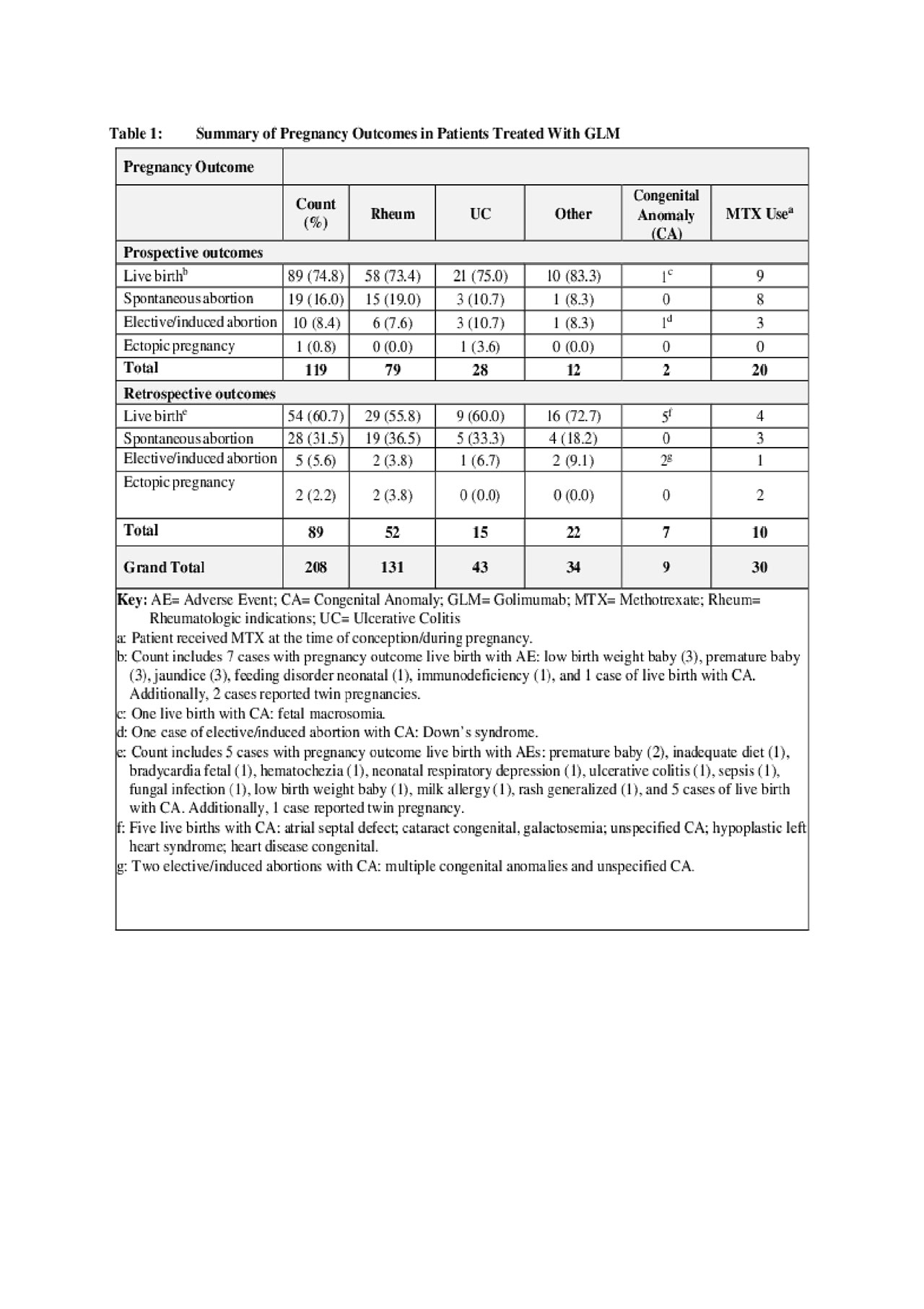
Results: Two hundred eight pregnancy cases[1] (131 rheumatological[2]; 43 UC; and 34 other) with 211 reported birth outcomes were identified. Of these 208 pregnancy cases, 119 were prospective and 89 were retrospective (Table 1). Average maternal age was 31.9 years. Of the 119 prospectively reported pregnancy cases, 89 (74.8%) resulted in live births, 19 (16.0%) resulted in spontaneous abortion (of these, 42.1% (8/19) received GLM in combination with methotrexate [MTX]), 10 (8.4%) resulted in induced/elective abortion, and 1 (0.8%) resulted in ectopic pregnancy. Overall, 9 congenital anomalies were reported (2 prospective/7 retrospective cases).
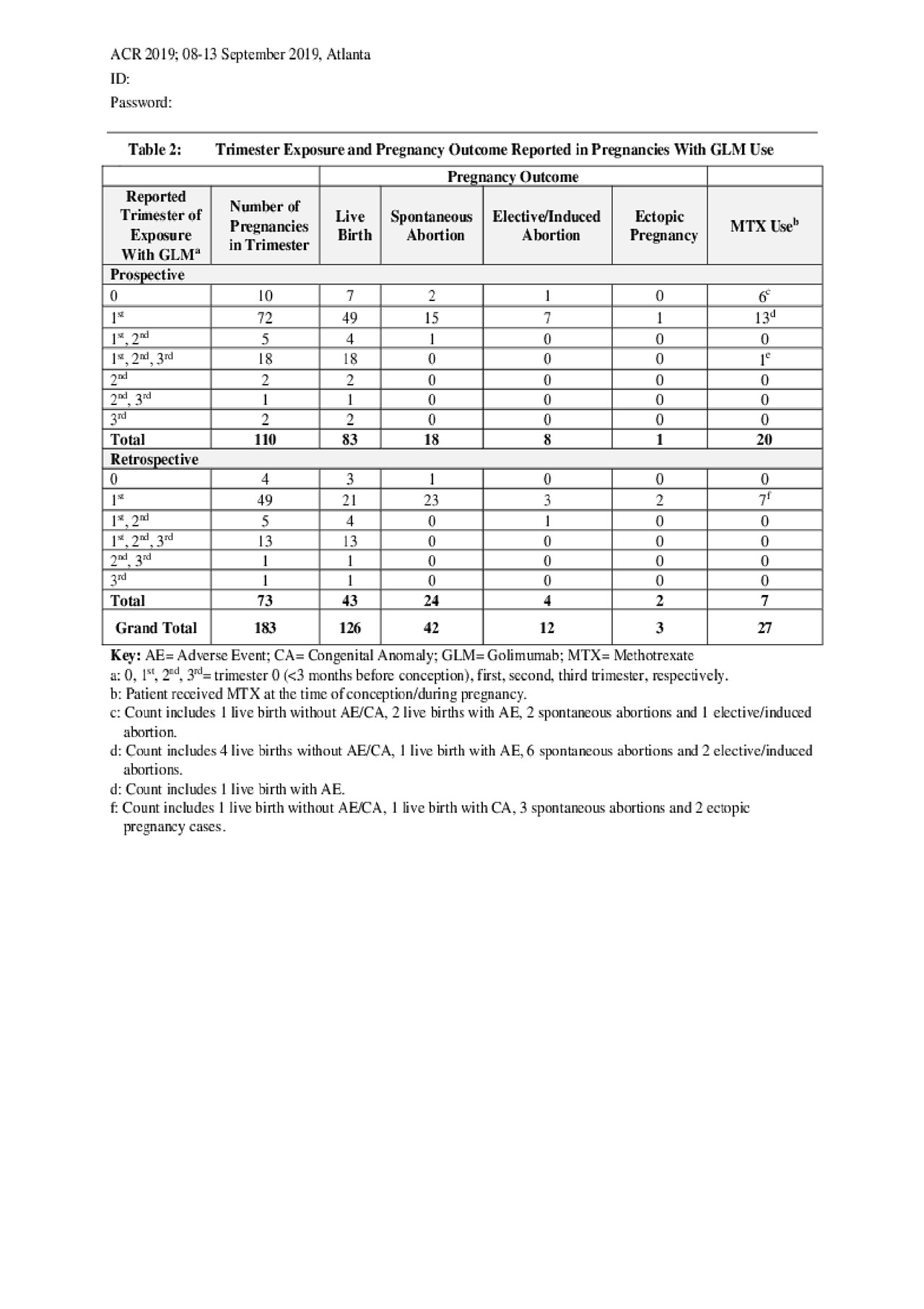
For 183 of the 208 pregnancy cases with reported outcomes, the trimester of exposure to GLM was known (Table 2). Among the 110 prospectively reported cases, 82 (74.5%) were exposed during trimester 0 or 1. Of these, 19 had concomitant exposure to MTX, with the following birth outcomes: 8 live births, 8 spontaneous abortions, 3 elective/induced abortions. Eighteen of the prospectively reported cases (16.4%) were exposed to GLM throughout pregnancy (1st, 2nd and 3rd trimester) and all resulted in live births.
[1] Three cases reported twin pregnancies.
[2] Rheumatological includes rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
Conclusion:
The rates of congenital malformations and spontaneous abortions were consistent with published background rates for the general population. Persistent exposure throughout pregnancy was rare, but not associated with apparent clinical sequelae. Limitations of this analysis include the lack of a direct comparison group, the variable amount of data available in the reports, and the possible bias towards reporting more negative outcomes in retrospective cases.
To cite this abstract in AMA style:
Esslinger S, Gabriel S, Otero-Lobato M, Clark M, Sheridan P, Geldhof A. Pregnancy Outcomes in Women Exposed to Golimumab [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/pregnancy-outcomes-in-women-exposed-to-golimumab-2/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pregnancy-outcomes-in-women-exposed-to-golimumab-2/