Session Information
Date: Monday, November 13, 2023
Title: (1100–1123) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Pegloticase can lower serum urate (SU) in patients with uncontrolled gout who are refractory to/intolerant of oral urate-lowering therapies. However, antidrug antibodies (ADAs) can limit pegloticase treatment response and put patients at risk for infusion reactions (IRs).1,2 Co-administering methotrexate (MTX) with pegloticase attenuates ADAs, resulting in improved response rates (71% vs. 39% during Month 6) and decreased IR risk (4% vs. 31%).3 Here we examine potential baseline factors that may influence urate-lowering response to pegloticase.
Methods: These analyses included uncontrolled gout patients who received biweekly pegloticase (8 mg) in the Phase 3 pegloticase registration1 and MIRROR RCT2 trials. Patients were included if they had received ≥1 pegloticase infusion. Treatment response was defined as SU < 6 mg/dL for at least 80% of Month 6. A classification and regression tree (CART) analysis was performed in patients who did (MIRROR RCT MTX arm) and did not (Phase 3, MIRROR RCT PBO arm) receive MTX co-therapy with pegloticase. CART analyses split datasets based on data homogeneity, providing insight on outcome prediction factors. The following pre-treatment factors were considered: patient age, sex, weight, BMI, SU, and ADA status (positive/negative).
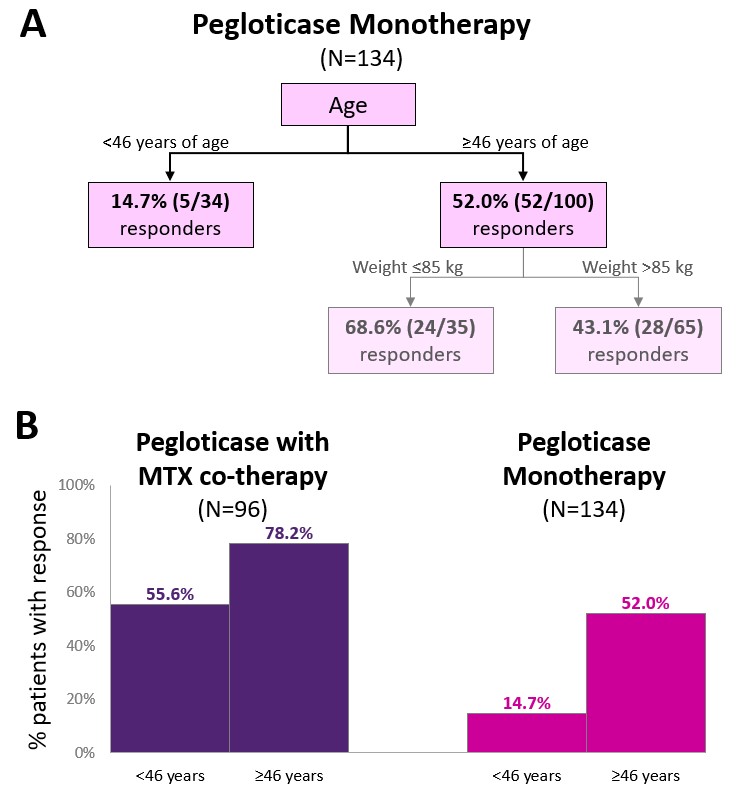
Results: Baseline characteristics in pegloticase monotherapy (N=134; age: 54.9±14.5 years, 82.1% men, BMI: 33.0±7.5 kg/m2, weight: 99.2±23.3 kg) and pegloticase+MTX (N=96; age: 56.0±12.5 years, 91.7% men, BMI: 32.8±5.6 kg/m2, weight: 102.6±19.4 kg) groups were similar. 57 monotherapy (42.5%) and 71 MTX (74.0%) patients were treatment responders. For the pegloticase monotherapy group, CART analysis selected an age cut-off of 46 years ( < 46 years: 14.7% response, ≥46 years: 59.0% response) as a first-level predictor and a body weight cut-off of 85 kg as a second level predictor (Figure, Part A). For the pegloticase+MTX group, CART analysis returned no decision tree. This finding indicates that no examined factors predicted pegloticase treatment response in the presence of MTX co-therapy. Although age did not predict treatment response in patients receiving MTX co-therapy, those ≥46 years of age had a higher response rate than those < 46 years of age (cutoff identified in the monotherapy group, 78% vs. 56%; Figure, Part B).
Conclusion: With pegloticase monotherapy, younger patient age and higher body weight were predictors of treatment failure. These findings are in agreement with pharmacokinetic studies showing an influence of ADAs and body surface area on serum pegloticase concentrations. However, in the presence of MTX-co-therapy, neither patient age nor body weight predicted response to pegloticase. These findings further support the importance of MTX co-administration with pegloticase to maximize the number of patients who may benefit from this often last-line therapy.
To cite this abstract in AMA style:
Mossell J, Duong M, Obermeyer K, Padnick-Silver L, LaMoreaux B, Chabra S. Predictors of Pegloticase Urate-lowering Response in the Presence and Absence of Methotrexate Co-therapy [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/predictors-of-pegloticase-urate-lowering-response-in-the-presence-and-absence-of-methotrexate-co-therapy/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-pegloticase-urate-lowering-response-in-the-presence-and-absence-of-methotrexate-co-therapy/