Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: ORAL Shift, a global Phase 3b/4 non-inferiority study, demonstrated sustained efficacy/safety of tofacitinib modified-release (MR) 11 mg QD following MTX withdrawal in patients (pts) with RA who achieved CDAI low disease activity (LDA) after treatment with tofacitinib + MTX.1 Here, we assessed predictors of durable clinical response in pts receiving tofacitinib MR 11 mg QD in ORAL Shift.
Methods: ORAL Shift (NCT02831855) enrolled pts aged ≥ 18 years with moderate to severe RA and an inadequate response to MTX. Pts received open-label (OL) tofacitinib MR 11 mg QD + MTX for 24 weeks. Pts achieving LDA (CDAI score ≤ 10) at W24 entered the 24-week double-blind (DB) MTX withdrawal phase and were randomized 1:1 to receive tofacitinib MR 11 mg QD + placebo (PBO) (tofacitinib monotherapy; ie blinded MTX withdrawal) or continue tofacitinib + MTX. In this post hoc analysis of randomized pts, we assessed predictors of durable response (maintenance of response from W24–48) per CDAI LDA and remission criteria (CDAI score ≤ 2.8). All covariates were initially assessed for significance in a univariate logistic regression. Highly correlated covariates were reviewed to assess which would be removed prior to modeling in a multivariable logistic regression. Remaining significant (p≤ 0.10) covariates in the univariate regression were selected in the model using a stepwise selection process with p≤ 0.15 entry and p≤ 0.05 stay criteria. From the final model, estimated odd ratios (ORs) with 95% confidence intervals (CIs) are presented.
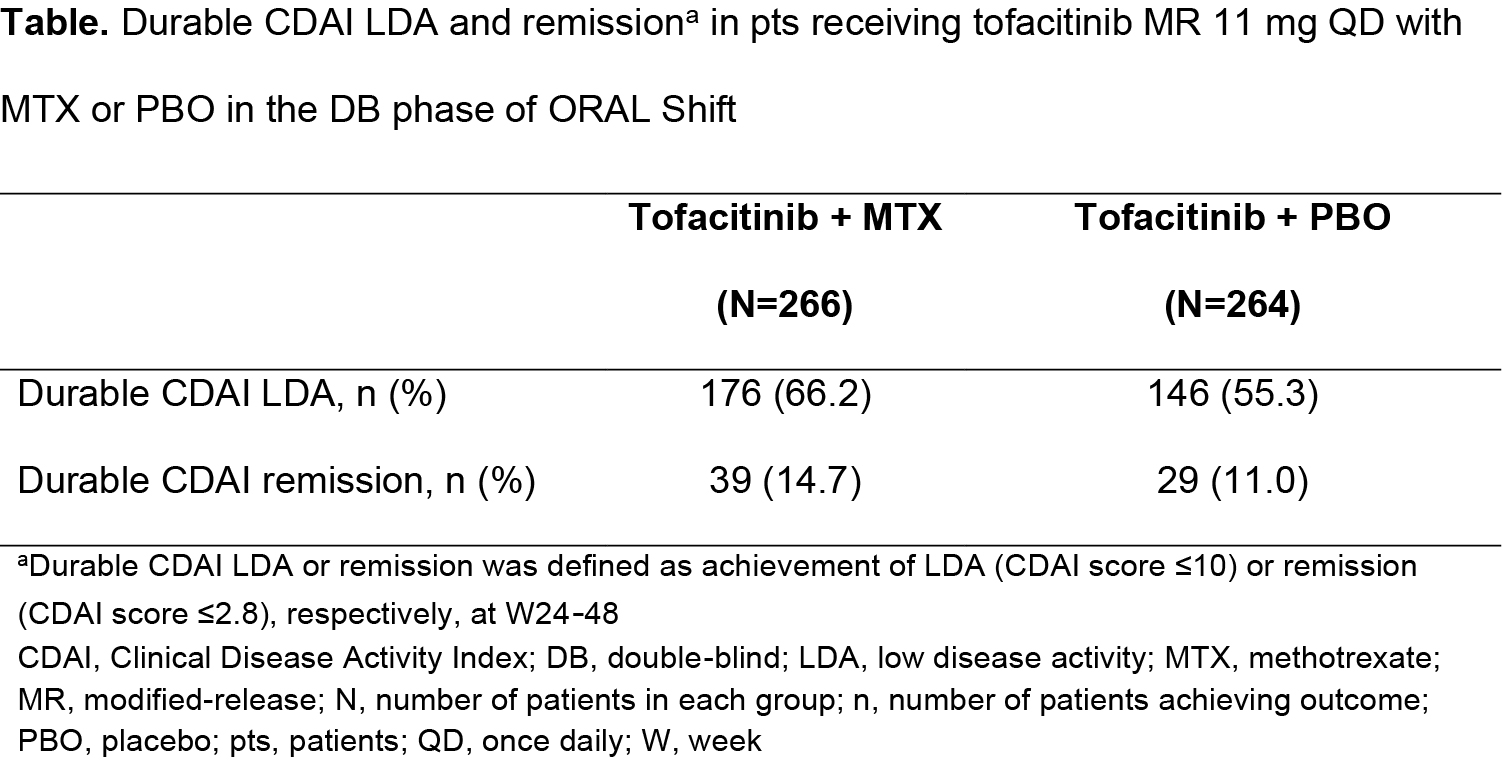
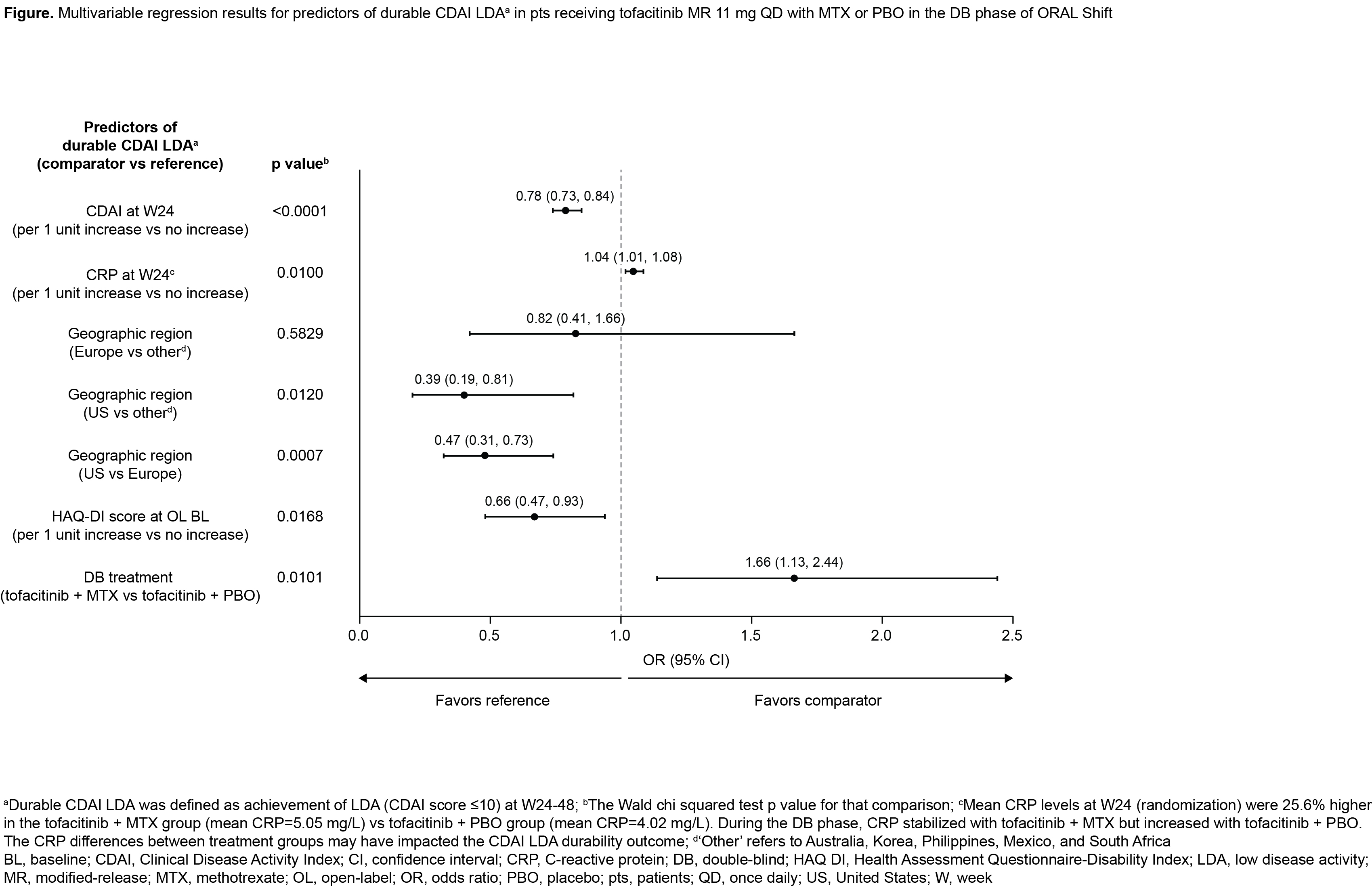
Results: In the DB phase of ORAL Shift, durable CDAI LDA and remission rates were: 66.2% and 14.7%, respectively, with tofacitinib + MTX (N=266); and 55.3% and 11.0%, respectively, with tofacitinib + PBO (N=264) (Table). In the multivariable analysis, five pt covariates significantly predicted durable CDAI LDA (Figure; discussed hereafter). Each unit increase in CDAI score at W24 reduced the likelihood of maintaining CDAI LDA by 22.0%. Each unit increase in CRP at W24 increased the likelihood of maintaining CDAI LDA by 4.0%; this may have been due to imbalanced CRP levels at W24 (randomization) between treatment groups (Figure, footnote d). The odds of durable CDAI LDA were 53.0% lower in the US vs Europe and 61.0% lower in the US vs ‘other’ regions. Each unit increase in baseline (BL) HAQ-DI score reduced the odds of durable CDAI LDA by 34.0%. Pts receiving tofacitinib + MTX had 66.0% greater odds of durable CDAI LDA vs pts receiving tofacitinib + PBO. CDAI at W24 was the only significant predictor of durable CDAI remission in the multivariable analysis: OR (95% CI) 0.32 (0.24, 0.43); p< 0.0001. Each unit increase in CDAI score at W24 reduced the odds of durable CDAI remission by 68.0%.
Conclusion: This post hoc analysis of data from ORAL Shift found that CDAI and CRP at W24, geographic region, BL HAQ-DI, and treatment could be predictors for durable CDAI LDA. As the findings of this post hoc analysis were limited to pts who achieved CDAI LDA at W24 with tofacitinib MR 11 mg QD + MTX, additional data in the general pt population need to be investigated.
- Cohen SB et al. Lancet Rheumatol 2019; 1: E23-E34.
Acknowledgments:
Study sponsored by Pfizer Inc. Medical writing support was provided by Sarah Piggott, CMC Connect, and funded by Pfizer Inc.
To cite this abstract in AMA style:
Yamaoka K, Cohen S, Sugiyama N, Shi H, Rivas J, Diehl A, Smolen J. Predictors of Durable Clinical Response to Tofacitinib 11 Mg Once Daily with or Without Methotrexate in Patients with Rheumatoid Arthritis: Post Hoc Analysis of Data from a Phase 3b/4 Methotrexate Withdrawal Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/predictors-of-durable-clinical-response-to-tofacitinib-11-mg-once-daily-with-or-without-methotrexate-in-patients-with-rheumatoid-arthritis-post-hoc-analysis-of-data-from-a-phase-3b-4-methotrexate-wit/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-durable-clinical-response-to-tofacitinib-11-mg-once-daily-with-or-without-methotrexate-in-patients-with-rheumatoid-arthritis-post-hoc-analysis-of-data-from-a-phase-3b-4-methotrexate-wit/