Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: CLIPPER is an ongoing, 8-year, phase 3b, multicenter, open-label study of the safety and efficacy of etanercept in the treatment of juvenile idiopathic arthritis (JIA) categorized as extended oligoarticular arthritis (eoJIA), enthesitis-related arthritis (ERA), or psoriatic arthritis (PsA). The aim of this study was to identify predictors of sustained 6-month clinical remission on medication using long-term data from CLIPPER.
Methods: Previously reported baseline characteristics of the 127 children enrolled in CLIPPER (60 eoJIA [2–17 years], 38 ERA [12–17 years], and 29 PsA [12–17 years])1 were analyzed post hoc as possible predictors of the attainment of clinical remission on medication (per the JIA ACR criteria or Juvenile Arthritis Disease Activity Score 71-joint [JADAS] criteria) sustained for 6 consecutive months using univariate logistic regression models and stepwise multivariate models. Clinical response and disease activity status after 4, 8, and 12 weeks of treatment were also evaluated as predictors. Analyses were based on observed cases in CLIPPER and 6-year follow-up data from the CLIPPER2 extension.
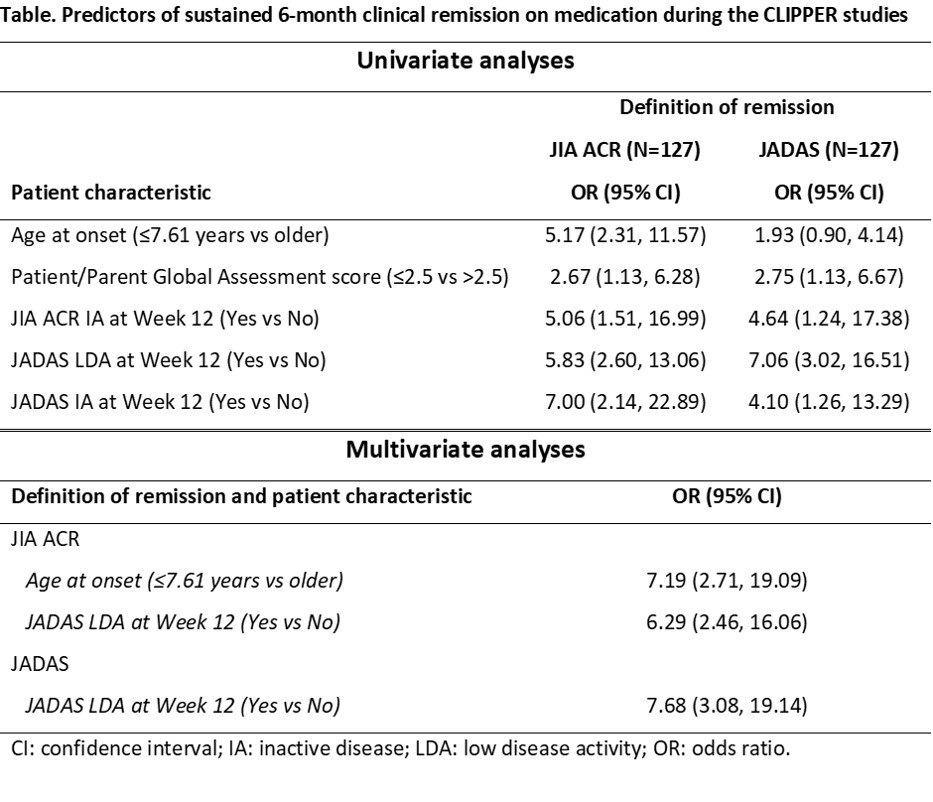
Results: Univariate analyses showed that baseline Patient/Parent Global Assessment score, JIA ACR inactive disease (IA) at Week 12, JADAS low disease activity (LDA) at Week 12, and JADAS IA at Week 12 were associated with the attainment of 6-month remission according to both JIA ACR criteria and JADAS criteria (Table). Multivariate analyses showed that age at onset and JADAS LDA at Week 12 were predictors of 6-month remission according to JIA ACR criteria, whereas JADAS LDA at Week 12 was a predictor according to JADAS criteria.
Conclusion: JADAS LDA at Week 12 of etanercept treatment was a predictor of attaining sustained 6-month clinical remission on medication according to JIA ACR criteria and JADAS criteria during the CLIPPER studies. Younger age at onset was also a predictor according to JIA ACR criteria.
References
1. Horneff G, et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann Rheum Dis. 2014;7:1114–22.
To cite this abstract in AMA style:
Vojinovic J, Chasnyk V, Dehoorne J, Panaviene V, Akikusa J, Avcin T, Chaitow J, Lauwerys B, Antón J, Penades I, Flato B, Boteanu A, Huppertz H, Jaller J, Graham D, Borlenghi C, Vlahos B, Zang C, Ruperto N. Predictors of Clinical Remission in Children with Extended Oligoarticular Arthritis, Enthesitis-related Arthritis, or Psoriatic Arthritis Treated with Etanercept in the CLIPPER Studies [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/predictors-of-clinical-remission-in-children-with-extended-oligoarticular-arthritis-enthesitis-related-arthritis-or-psoriatic-arthritis-treated-with-etanercept-in-the-clipper-studies/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-clinical-remission-in-children-with-extended-oligoarticular-arthritis-enthesitis-related-arthritis-or-psoriatic-arthritis-treated-with-etanercept-in-the-clipper-studies/