Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: With increasing use of biologic disease modifying anti-rheumatic drugs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) for the management of rheumatoid arthritis (RA), outcomes have improved for many patients. In previous studies, factors associated with use of bDMARDs/tsDMARDs have varied considerably. The aim of this study was to investigate the relation between patient characteristics at RA diagnosis and subsequent initiation of treatment with bDMARDs/tsDMARDs.
Methods: Patients with early RA (symptom duration < 12 months) diagnosed at the outpatient clinics of a University Hospital in 2012-2016 who fulfilled established classification criteria for RA (the 1987 ACR criteria and/or the 2010 ACR/EULAR criteria) were included. Patients who participated in a clinical trial were excluded. Clinical data were retrieved from the medical records in a structured review process. Data on treatment were obtained from a national quality register, and from complementary case record reviews. Data on comorbidities were retrieved from a regional healthcare register, and data on level of formal education, country of origin and family background from national registers. The relation between characteristics at diagnosis and time to start of a first bDMARD/tsDMARD was examined in crude and age-adjusted Cox regression models. Patients were censored at death, migration from Sweden or end of the study (December 31, 2021). Factors with p< 0.20 in crude models were eligible for multivariable analyses. In cases of major collinearity (r >0.3), only the covariate with the strongest association in crude analysis was included in the multivariable model.
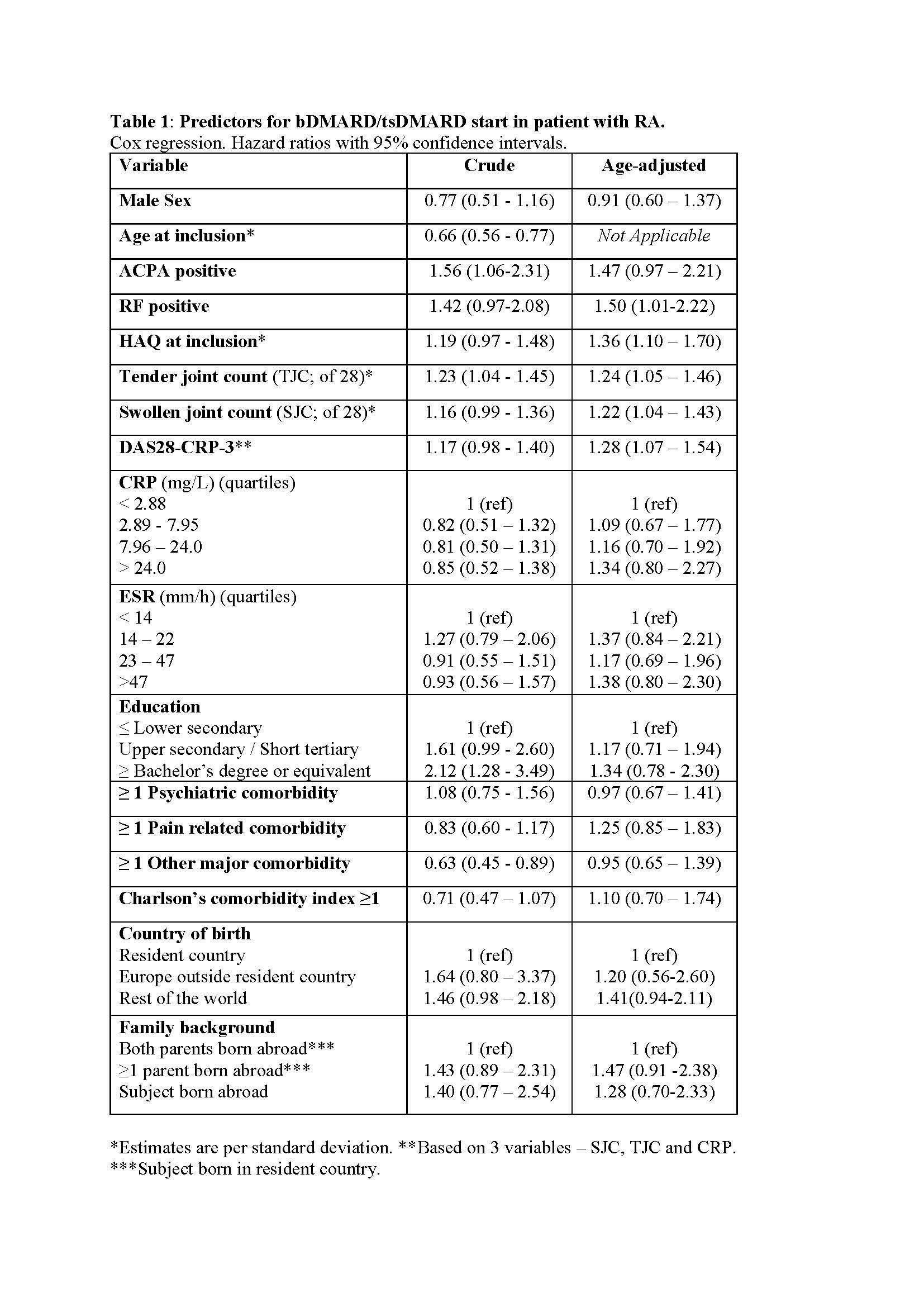
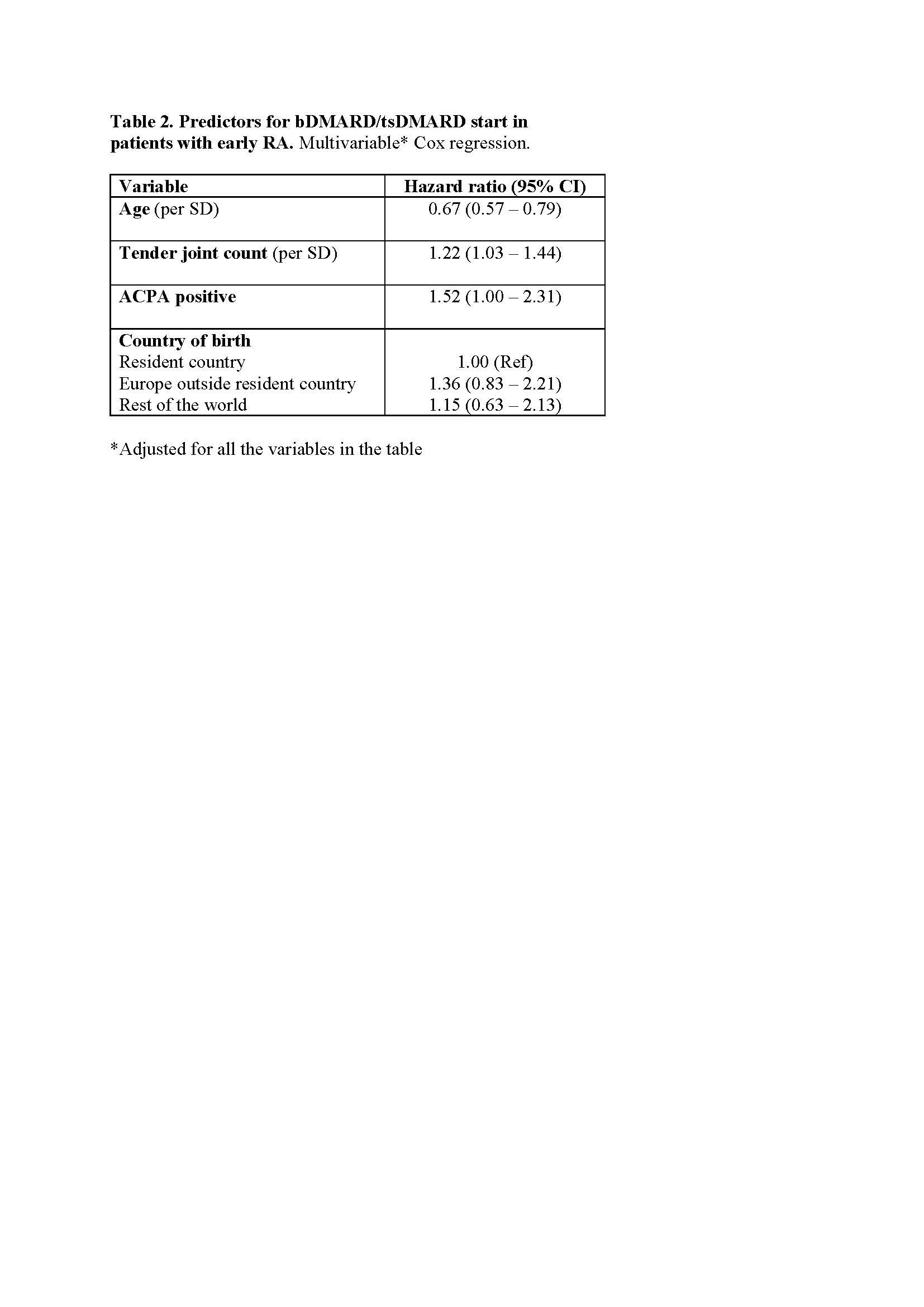
Results: A total of 367 patients with early RA were identified. As 37 of those participated in a clinical trial, 330 patients (mean age 59.3 years; 72 % women; 68 % anti-citrullinated protein antibody (ACPA) positive) were included. Treatment with a first bDMARD was initiated in 137 patients (41 %) during the follow-up, and never preceded by tsDMARD initiation. Higher age at diagnosis was associated with a lower probability of starting a bDMARD (hazard ratio 0.66 per SD; 95% confidence interval 0.56-0.77). Positive ACPA and baseline disease severity, measured by tender or swollen joint counts, or the Health Assessment Questionnaire (HAQ), but not by CRP or ESR, were associated with subsequent bDMARD treatment initiation (Table 1). A high level of formal education and absence of comorbidities predicted start of a bDMARD in crude, but not in age-adjusted, analyses. The association with age remained significant in models adjusted for each other covariate separately. In the final multivariable model, lower age, positive ACPA and many tender joints at diagnosis were all predictive of bDMARD treatment (Table 2).
Conclusion: ACPA positive patients with RA, and patients with extensive joint involvement or substantial disability at diagnosis, were more likely to receive early treatment with bDMARDs, whereas older patients were less likely to start bDMARDs. The impact of age on bDMARD therapy was not explained by level of education, comorbidities or ethnicity, suggesting that other aspects of age influence treatment decisions in early RA.
To cite this abstract in AMA style:
Hameed M, Exarchou S, Eberhard A, Sharma A, Bergström U, Einarsson J, Turesson C. Predictors at Diagnosis for Start of Biologic Disease Modifying Anti-Rheumatic Drugs in Patients with Early Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/predictors-at-diagnosis-for-start-of-biologic-disease-modifying-anti-rheumatic-drugs-in-patients-with-early-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-at-diagnosis-for-start-of-biologic-disease-modifying-anti-rheumatic-drugs-in-patients-with-early-rheumatoid-arthritis/