Session Information
Date: Monday, November 18, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Pegloticase is a pegylated recombinant uricase approved for treatment of chronic uncontrolled gout. Because of the development of anti-drug antibodies (ADA), persistent urate lowering responsiveness is limited to approximately 42% of patients. Factors contributing to the lack of responsiveness have not been identified, however, making it difficult to predict individuals likely to be persistent responders.
Methods: A total of 225 subjects from the phase 3 trials of pegloticase in chronic uncontrolled gout were analyzed1. Unresponsiveness was defined as failure to have a serum urate < 6 mg/dL during 80% of the measurements after 6 months of treatment. For this analysis, plasma urate (PUA) was employed to avoid artifact related to the presence of active pegloticase in blood samples. Logistic regression and propensity models with response and non-response as the classes were developed using the following variables: gender, age, baseline BMI, weight (kg), diabetic status, presence of tophi, ever used methylprednisolone, Fib-4, creatinine, PUA and pegloticase levels.
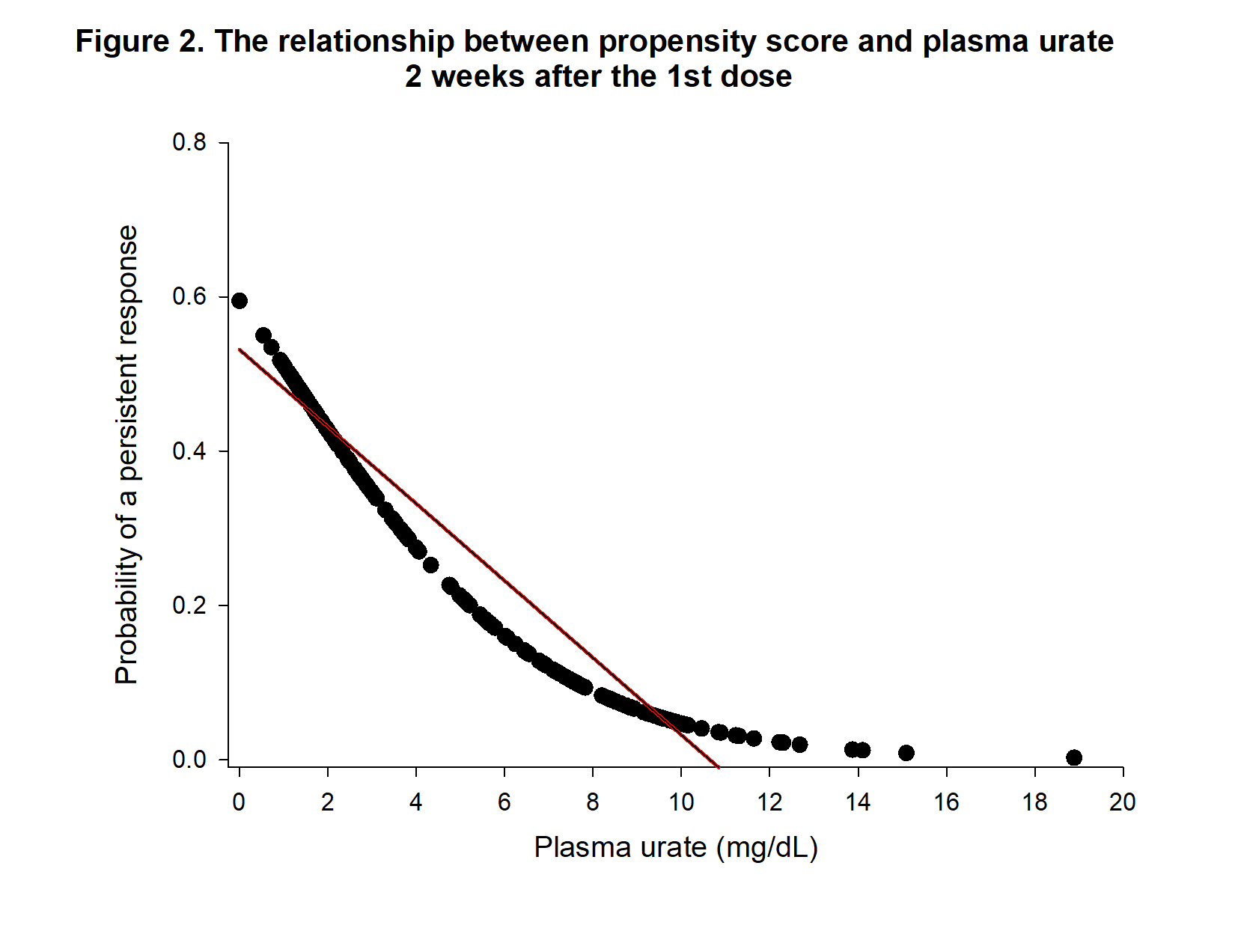
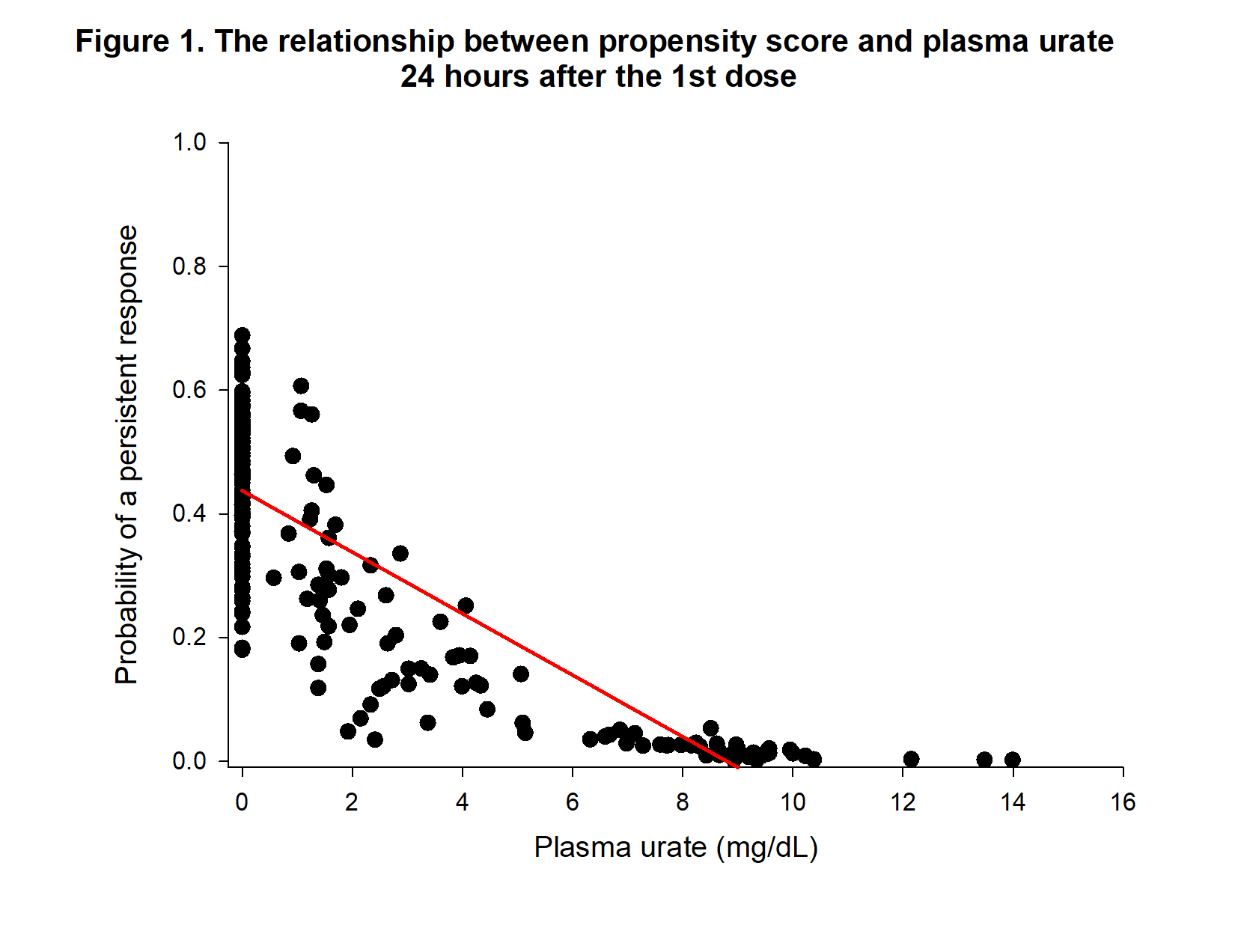
Results: First, we developed a logistic regression model based on information assessed 24 hours after the initial dose of pegloticase to avoid the effect of ADA that usually develops later in treatment 2. Of all the variables, logistic modelling indicated that baseline weight (p = 0.0133) and 24 hr PUA (p = 0.0008) were significantly related to persistent responsiveness. Relating 24 hr PUA to the propensity score yielded a significant (p < 0.0001) relationship: Propensity score = 0.43733 -0.04970* PUA indicating that lower 24 hr PUA is associated with persistent response (Figure 1). Because monitoring of pegloticase treatment usually occurs 2 weeks after initial treatment, we also generated a logistic regression model employing data obtained at this time. The logistic regression model using the data at 2 weeks post initial pegloticase treatment showed that only PUA ( p< 0.0001) was significant. The relationship between 2 week PUA to the propensity score is described by Propensity score = 0.53161 -0.04995*PUA (p< 0.0001), indicating that lower PUA values are associated with persistent response (Figure 2).
Conclusion: Measurement of PUA at 24 hours and 2 weeks post initial treatment provides information that can predict persistent urate lowering in patients with chronic uncontrolled gout. Weight is associated with 24 hr PUA levels, suggesting that weigh-based dosing could be useful to increase responsiveness to pegloticase.
To cite this abstract in AMA style:
Lipsky P, yeo a. Prediction of the Response of Patients with Chronic Uncontrolled Gout to Pegloticase [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/prediction-of-the-response-of-patients-with-chronic-uncontrolled-gout-to-pegloticase/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prediction-of-the-response-of-patients-with-chronic-uncontrolled-gout-to-pegloticase/