Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Secukinumab (SEC) proved to be an effective treatment for patients with psoriatic arthritis (PsA) in previous randomized clinical trials [1]. There is only limited knowledge on prediction of low disease activity (LDA) and treatment strategy in PsA patients under SEC treatment in routine clinical care.
Using real-world data from the German non-interventional study AQUILA [2], the main objectives were (1) to predict LDA in individual PsA patients treated with SEC through machine learning methods and (2) to identify the most important predictors and their influence on the prediction using explainable artificial intelligence (XAI).
Methods: Data of 1041 PsA patients from the AQUILA study were used. Thirty-three demographic, clinical and treatment parameters at baseline (BL) served as input data to develop prediction models. LDA was defined as physician global assessment (PhGA) ≤ 2 at week (w) 16 (+/- 6 w). Samples were divided into training (70%) and validation (30%) cohorts. Ten different prediction models were applied and compared. Model performance was measured using area under the receiver operating characteristic curve (AUROC) which represents the probability that a randomly selected patient with LDA will have higher prediction to achieve LDA than a patient with moderate/high disease activity. Additionally, sensitivity and specificity of the prediction model were computed and express the proportion of correctly identified patients who reach or don’t reach LDA at w16, respectively. Shapley XAI estimated importance and impact of each predictor based on how it affected the change in individual prediction [3].
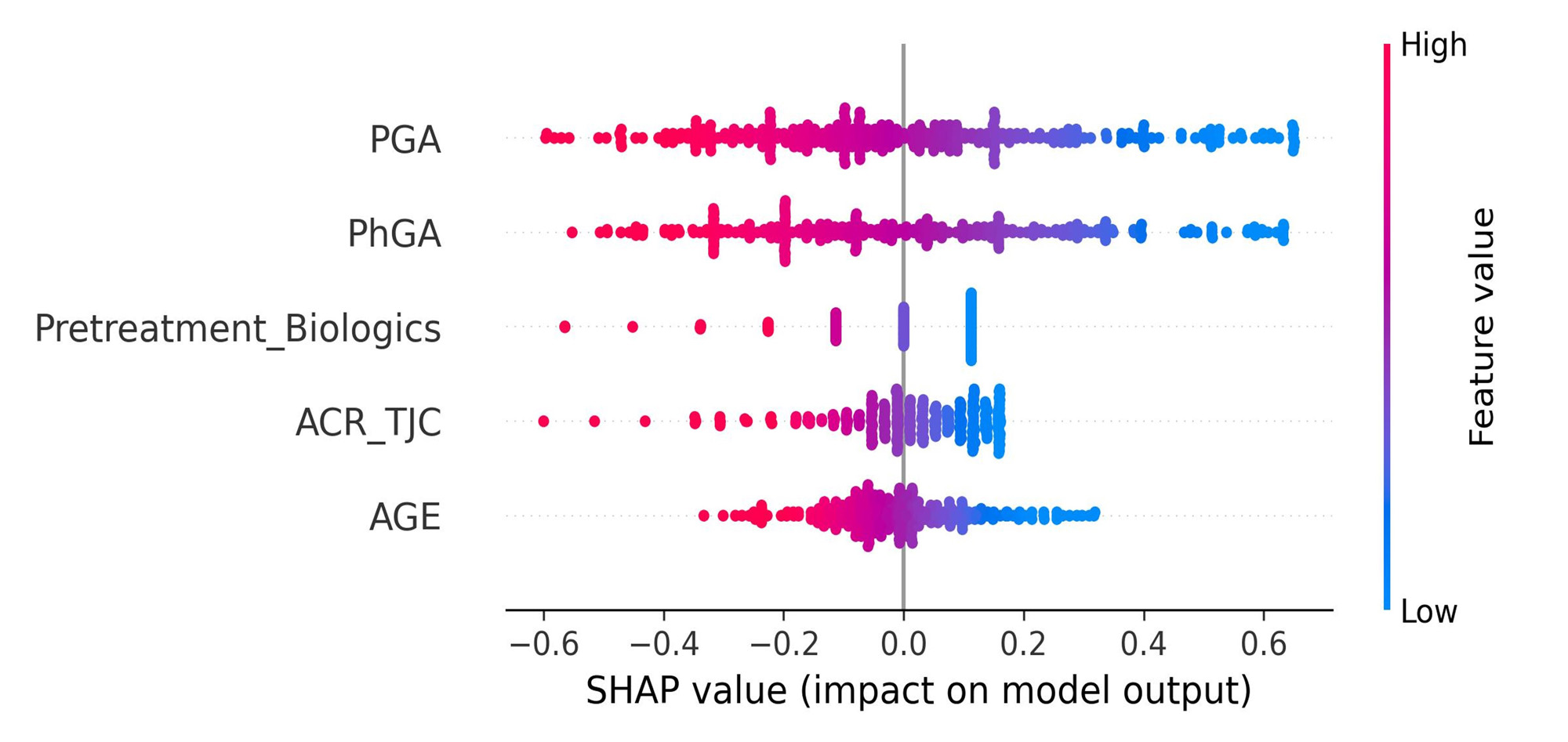
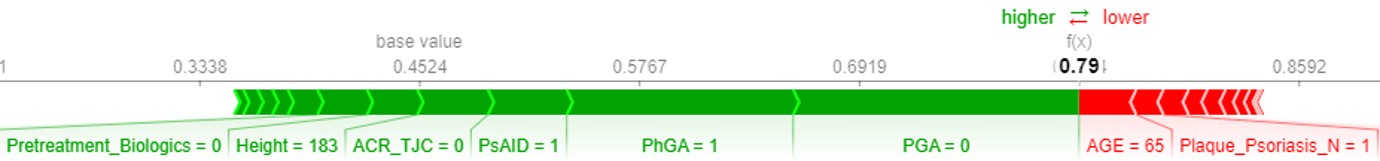
Results: The most influencing predictor was patient global assessment (PGA) at BL, followed by PhGA, number of pretreatments with biologics, tender joint count and age (Figure 1 A). AUROC of the best prediction model was 0.68 in validation cohort. Sensitivity and specificity were 0.62 and 0.64, respectively. Applied XAI approach showed that lower BL values of all main predictors have higher probability of reaching LDA at w16. The highest probability was evident in biologic-naive patients (Figure 1 A). The approach also provided visual explanations of patient-individual predictions: Variables with values shown in green color increased probability of reaching LDA at w16, whereas red ones showed the opposite effect (Figure 1 B).
Conclusion: A promising prediction model accuracy of LDA in PsA patients treated with SEC could be reached and validated. Identified main predictors at BL, such as PGA and number of pretreatments with biologics, and their direction of influence on the prediction mostly match the existing clinical knowledge [4]. The analysis showed that XAI can provide useful clinical insights in patient-individual predictions, potentially guiding PsA treatment decisions in future.
To cite this abstract in AMA style:
Vodencarevic A, Brandt-Juergens J, Peterlik D, Gmeiner B, Kiltz U. Prediction of Low Disease Activity in Patients with Psoriatic Arthritis Treated with Secukinumab in Real World – Data from a German Observational Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/prediction-of-low-disease-activity-in-patients-with-psoriatic-arthritis-treated-with-secukinumab-in-real-world-data-from-a-german-observational-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prediction-of-low-disease-activity-in-patients-with-psoriatic-arthritis-treated-with-secukinumab-in-real-world-data-from-a-german-observational-study/