Session Information
Date: Monday, November 8, 2021
Title: RA – Treatments Poster II: PROs, Biomarkers, & Systemic Inflammation (1223–1256)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: A treat-to-target approach for RA management is recommended, with the aim of achieving remission.1,2 The Abatacept SubCutaneOus in Routine clinical practicE (ASCORE; NCT02090556) study assessed efficacy and safety of subcutaneous (SC) abatacept for the treatment of patients with moderate-to-severe active RA; month 12 retention and clinical response rates were better in patients receiving abatacept as a first- versus later-line biologic (b)DMARD.3 This post hoc analysis of ASCORE investigated the association of baseline variables with the achievement of DAS28 (CRP), Simplified Disease Activity Index (SDAI), and Clinical Disease Activity Index (CDAI) remission following treatment with SC abatacept in patients with RA.
Methods: Patients from ASCORE (recruited between February 2013 and April 2017) who initiated SC abatacept 125 mg once weekly were included. Analysis of baseline factors predictive of DAS28 (CRP; < 2.6), SDAI (≤ 3.3), and CDAI (≤ 2.8) remission at month 12 was conducted by treatment line (first- and ≥ second-line) and in the overall population. Variables found to be significant (P < 0.2) in univariate logistic regression analysis were selected as covariates in a multivariate backward elimination logistic regression model. Odds ratios (95% confidence intervals) and P values were calculated. Standardized values were used for the continuous baseline predictors.
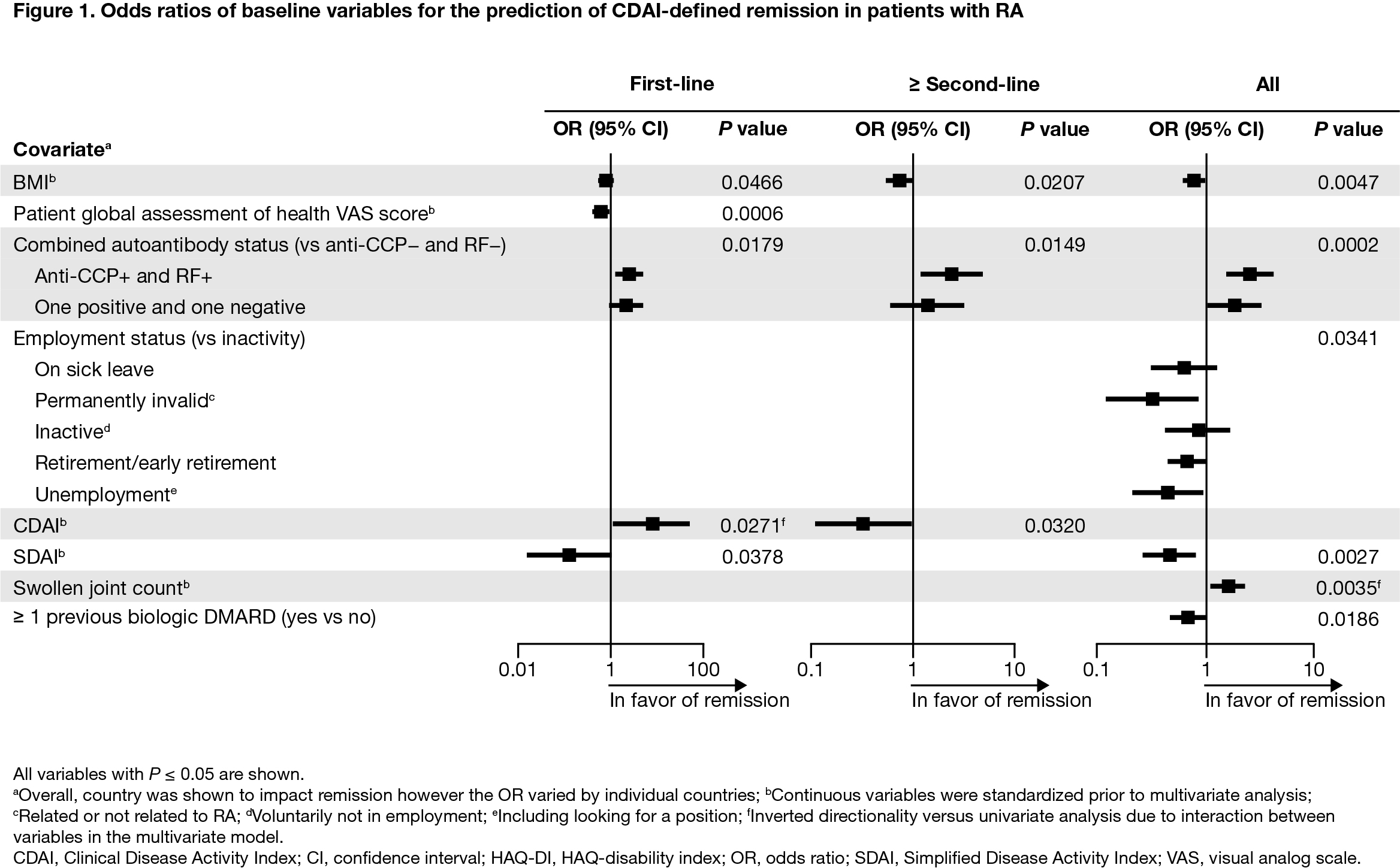
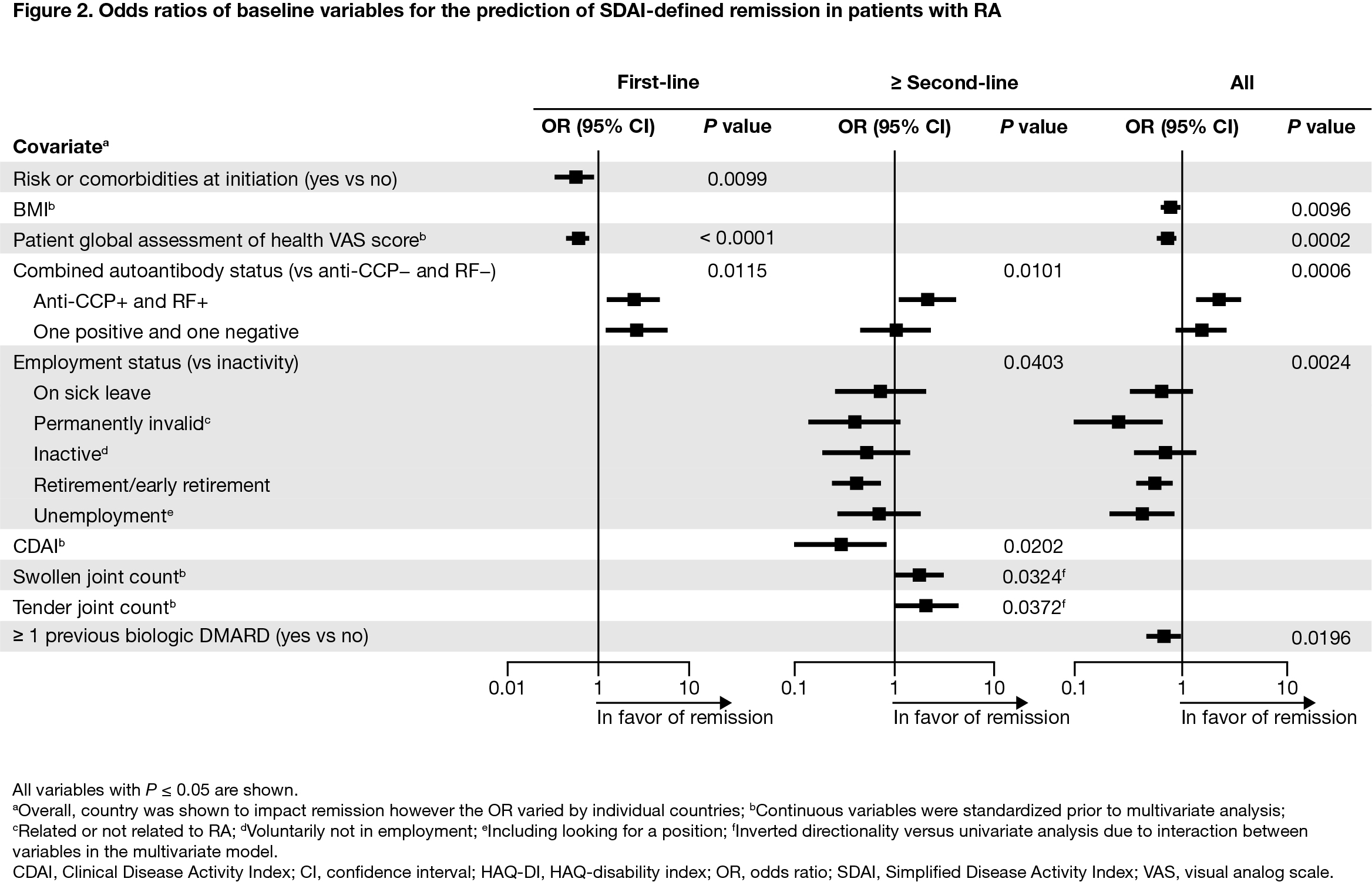
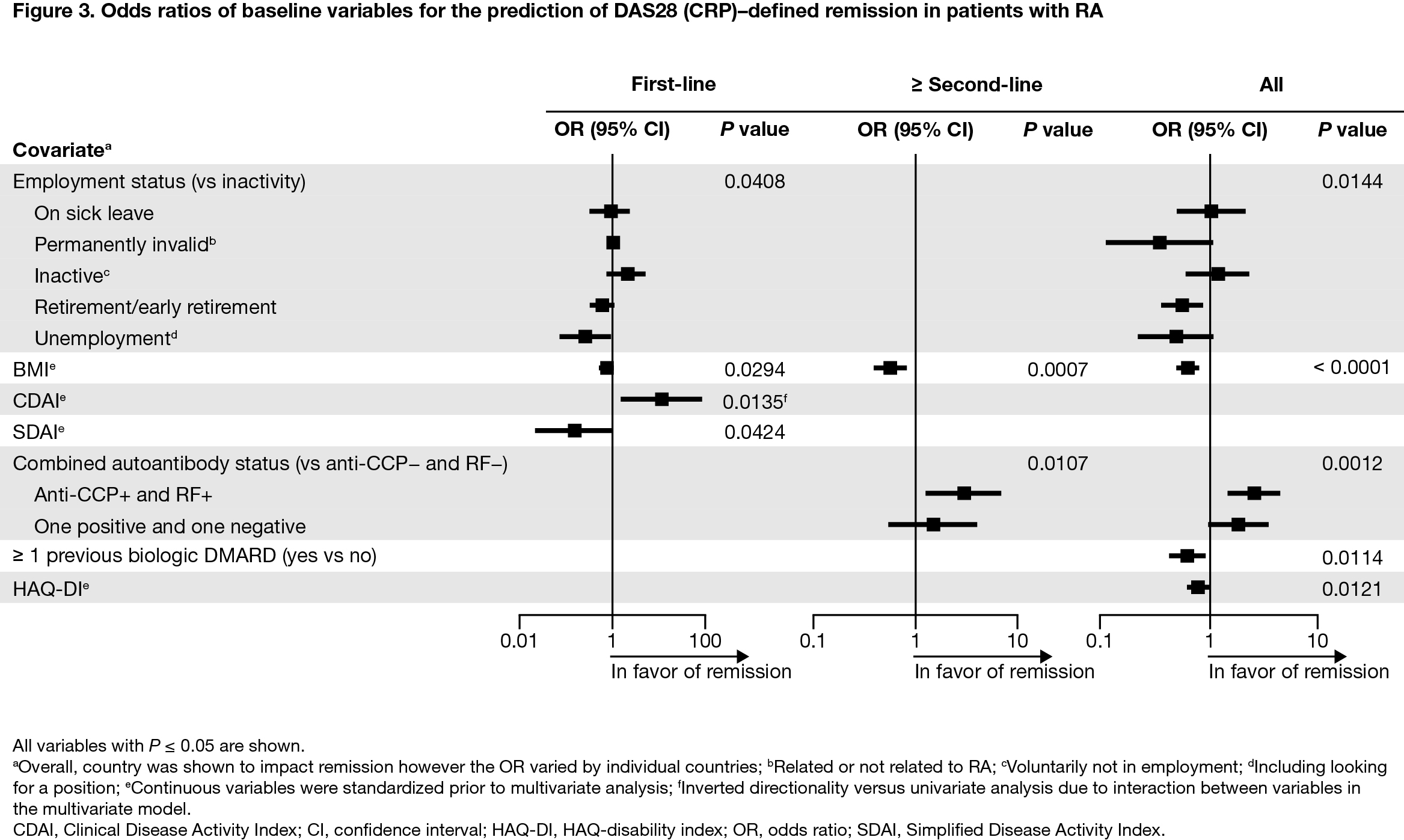
Results: Overall, 2892 patients were included (first-line, n = 1198; ≥ second-line, n = 1694). Baseline mean (standard deviation) age was 57.7 (12.7) years, BMI was 27.2 (5.8) kg/m2, and HAQ-disability index (DI) score was 1.4 (0.7); 78.6% were female, 61.7% were double seropositive (RF+ and ACPA+), and 18.6% were either RF+ or ACPA+. For patients treated with abatacept as first-line therapy, double/single seropositivity (vs negativity) was a predictor of CDAI (Figure 1) and SDAI remission (Figure 2). For patients treated with abatacept as ≥ second-line therapy, double seropositivity (vs negativity) was a predictor of DAS28 (CRP) (Figure 3), SDAI, and CDAI remission. For the overall population, double/single seropositivity (vs negativity) was a predictor of DAS28 (CRP), SDAI, and CDAI remission. Active (vs inactive) employment status, low BMI, no previous bDMARD use, and low HAQ-DI were predictive of achieving remission in the overall population.
Conclusion: In this post hoc analysis of the real-world ASCORE study, several baseline characteristics were associated with remission in patients with RA treated with SC abatacept. Patients with seropositive RA, no previous bDMARD use, low HAQ-DI, and low BMI at baseline were more likely to achieve remission when treated with abatacept. Seropositivity was associated with remission regardless of abatacept treatment line. Early identification of patients with seropositive RA may facilitate a precision medicine approach to treatment.
References:
1. Singh JA, et al. Arthritis Rheumatol 2016;68:1–26.
2. Smolen JS, et al. Ann Rheum Dis 2020;79:685–699.
3. Alten R, et al. Ann Rheum Dis 2019;78(Suppl 2):A1639.
Medical writing: Rachel Rankin, PhD (Caudex), funded by Bristol Myers Squibb
To cite this abstract in AMA style:
Alten R, Rauch C, Bannert B, Marsal S, Buch M, Caporali R, Chartier M, Connolly S, Griffiths H, Mariette X, Nurmohamed M, Patel Y, Peichl P, Sanmarti R, Elbez Y, Lozenski K. Predicting RA Remission with Subcutaneous Abatacept Treatment in the Real-world Setting [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/predicting-ra-remission-with-subcutaneous-abatacept-treatment-in-the-real-world-setting/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predicting-ra-remission-with-subcutaneous-abatacept-treatment-in-the-real-world-setting/