Session Information
Date: Monday, November 8, 2021
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Rheumatoid Arthritis (RA) is a chronic inflammatory condition often associated with joint destruction, disability, and reduced life expectancy. Before RA diagnosis, some patients may present with seropositive arthralgia characterised by pain and/or stiffness without any clinical evidence of inflammation.
Seropositive arthralgia patients RA defined as joint pain without swelling, positive anti-citrullinated peptide antibodies (ACPA) or IgM rheumatoid factor (RF) are ‘at risk’ of developing RA. In this study we examine the knee arthroscopy and synovial biopsy features of ‘at risk’ patients for evidence of subclinical inflammation and assess its ability to predict progression to RA.
Methods: Seropositive arthralgia patients underwent needle arthroscopy and synovial biopsy of a knee joint. . The degree of synovitis and vascularity were recorded on a 0-100mm visual analogue scale. The synovium was examined by routine histology of H&E staining. Patients were followed up at regular intervals (3 months, 6 months and1 year) with a clinical assessment and laboratory investigations to evaluate if they developed RA according to the ACR/EULAR 2010 criteria.
Results: A total of 46 patients were recruited, 78% developed RA. X length of follow up. Patient demographics are outlined in Table 1. Family history, smoking and early morning stiffness were not predictive of developing RA. A statistically significant correlation was found between ACPA levels of >340 and the development of RA (P=.03). A synovitis or vascularity score of greater than 50% at arthroscopy significantly correlated with RA development (P=.04; P=.002, respectively). If a patient had both synovitis and vascularity score of 50%, it was strongly associated with progression to RA (P< .001).
If treated with conventional synthetic(cs) DMARDs for arthralgia, the median time for developing RA was 12 months compared to 3 months in those who did not receive treatment. Figure 1 outlines time to developing RA. The clinical course of the patients over 12 months is outlined in Figure 2.
Conclusion: Seropositive arthralgia patients ‘At risk’ of developing RA demonstrate >50% macroscopic synovitis or vascularity score at knee arthroscopy. ACPA titre > 340 is associated with progression and csDMARD treatment may delay, but not prevent, onset of RA .
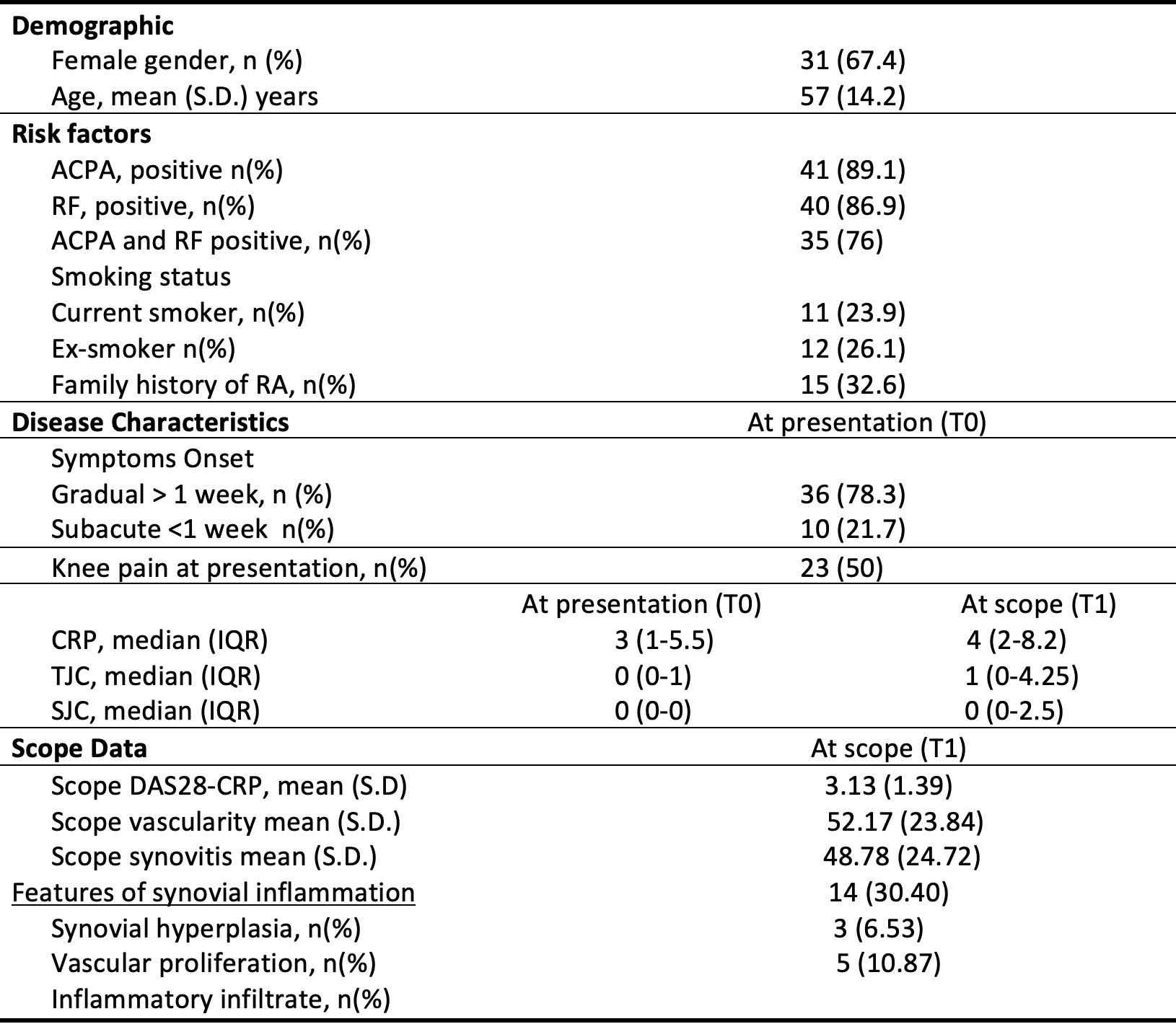 Table 1: Baseline demographics and scope data
Table 1: Baseline demographics and scope data
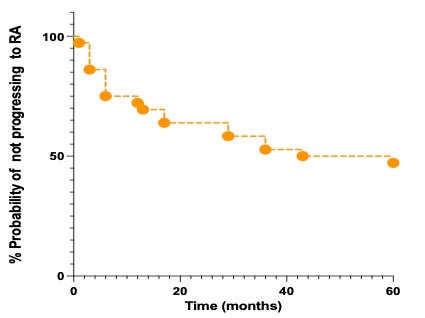 Figure 1: Kaplin-Meier Curve of the timeline of patients converting to RA
Figure 1: Kaplin-Meier Curve of the timeline of patients converting to RA
 Figure 2: Clinical course over 12 months from scope (T1)
Figure 2: Clinical course over 12 months from scope (T1)
To cite this abstract in AMA style:
Gorman A, Flynn K, Turk M, Low C, Murray K, Orr C, Gallagher P, Freeman L, Meaney E, Molloy E, O Neill L, Fearon U, Veale D. Predicting Progression to RA in Patients with Seropositive Arthralgia [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/predicting-progression-to-ra-in-patients-with-seropositive-arthralgia/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predicting-progression-to-ra-in-patients-with-seropositive-arthralgia/
