Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Nearly 20% of pregnancies in systemic lupus erythematosus (SLE) patients result in an adverse pregnancy outcome (APO); early identification of those at high APO risk is vital. We previously developed and cross-validated several regression and machine learning (ML) models for predicting APO using data from the PROMISSE Study, a large multi-center, multi-ethnic/racial study of APO in women with SLE and/or aPL. Penalized logistic regression (LASSO) and Super Learner were the best performing predictive algorithms with internal cross-validated area under the receiver operating curve (AUC) of 0.77-0.78. Our goal was to externally validate these two promising APO prediction models using six independent cohorts from the United States and Europe.
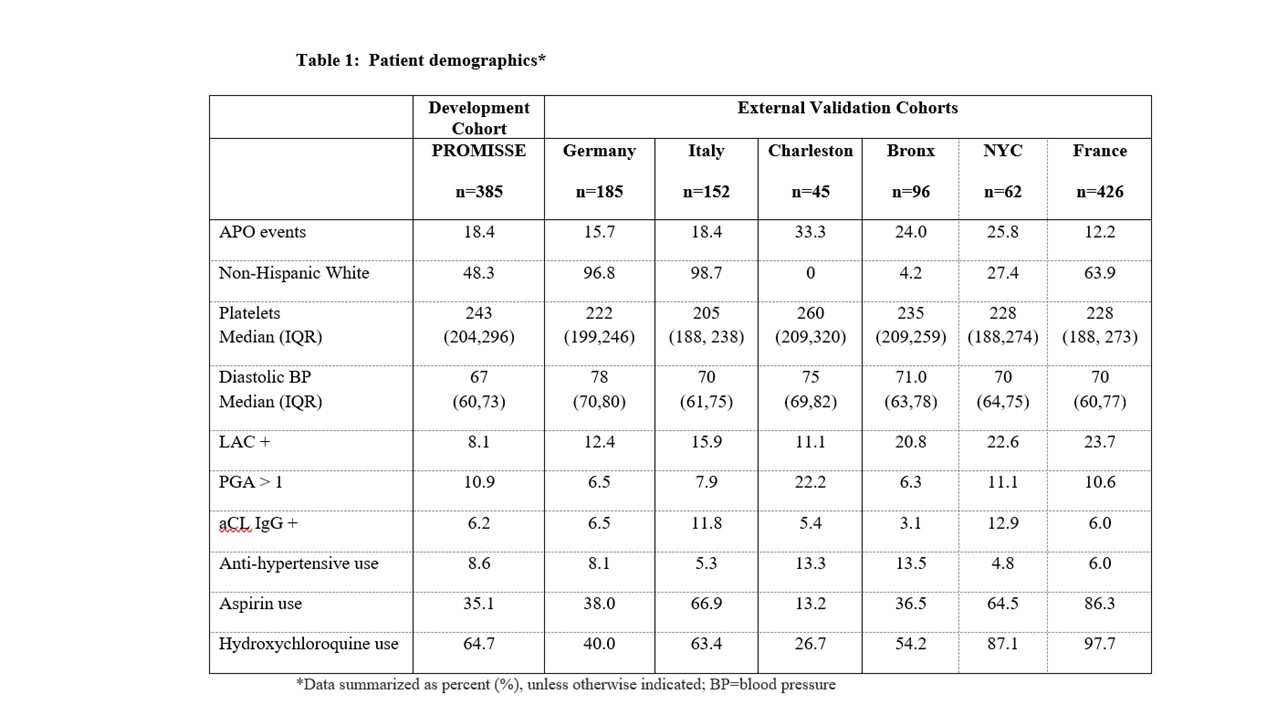
Methods: PROMISSE data consisting of 385 pregnancies, 81 APO events, and 32 known or potential risk factors measured in the first trimester of pregnancy was previously used to develop APO prediction models. APO was defined as preterm delivery due to placental insufficiency or preeclampsia, fetal or neonatal death, or fetal growth restriction. Six independent prospective cohorts of SLE pregnancies with no exclusion criteria from the US (Bronx and NYC, NY; Charleston, SC) and Europe (Italy, France, Germany) were available for external model validation. Missing data in each cohort were imputed using the MICE procedure. We assessed and compared cohort characteristics and performance of the APO prediction models in each cohort and in the combined data set using AUC to measure ability to discriminate patients with and without APO (AUC=1 implies perfect discrimination).
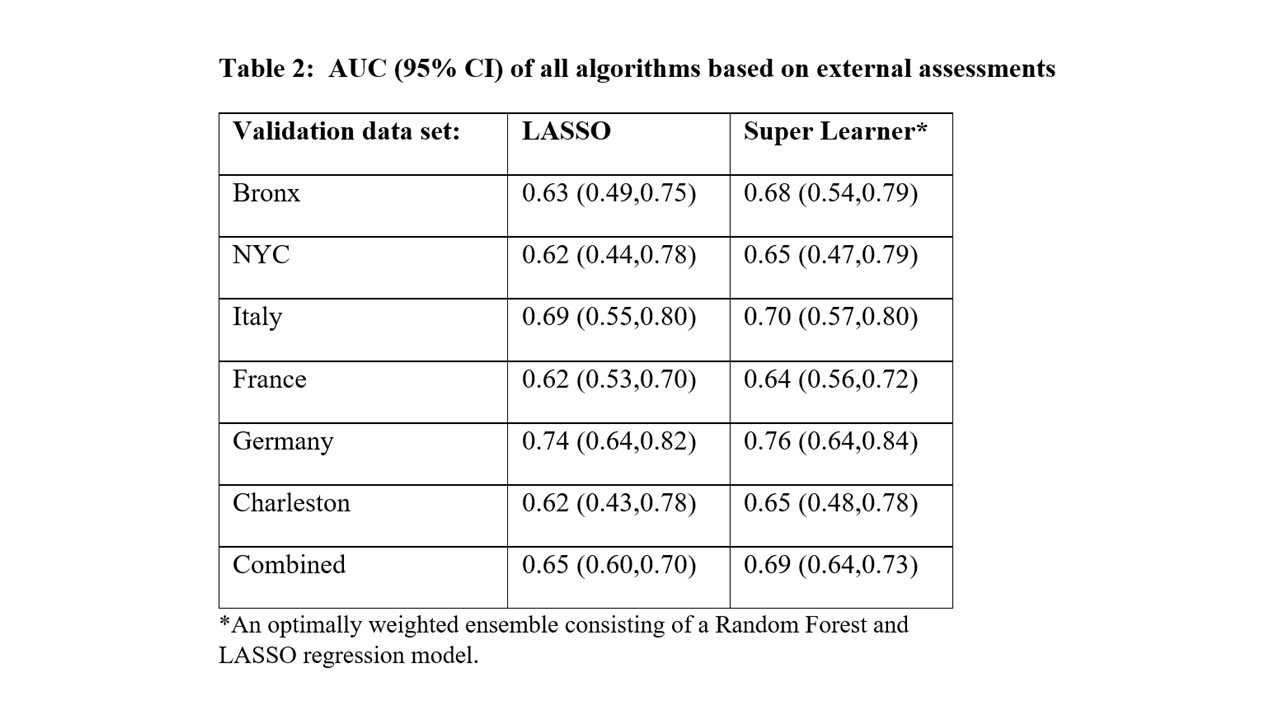
Results: The six external cohorts and PROMISSE showed distributional differences in APO risk factors (Table 1): the percentage of non-Hispanic White (NHW) ranged from 0% (Charleston) to nearly 100% (Germany, Italy); hydroxychloroquine use ranged from 27% (Charleston) to 98% (France) (Table 1). APO rates were similar in PROMISSE and Italy (18.4%), lower in France and Germany (12.2 and 15.7%), and higher in the Bronx (24%), Charleston (33%), and NYC (25.8%). Despite these differences in cohort characteristics, Super Learner for APO prediction developed using PROMISSE data performed well across external cohorts, with AUC from 0.64 (France) to 0.76 (Germany; Table 2). LASSO also maintained consistent external performance with AUC from 0.62 (NYC) to 0.74 (Germany). APO prediction models performed best in the Italy and Germany cohorts; both are larger cohorts with little missing data. Model performance was not associated with cohort location (U.S. vs. Europe) or racial distribution. Key APO risk factors identified in PROMISSE and confirmed in external cohorts included higher diastolic blood pressure, lower platelet counts, LAC positivity, anti-hypertensive use and PGA positivity.
Conclusion: APO prediction algorithms developed with PROMISSE data using variables obtained early in pregnancy were validated for discrimination in six external data sets, providing confirmation of geographic and temporal transportability. Further refinement of our APO prediction models will be explored along with model calibration in future studies.
To cite this abstract in AMA style:
Fazzari M, Salmon J, Guerra M, Costedoat-Chalumeau N, LE GUERN V, Guettrot-Imbert G, Mosca M, Zucchi D, Tani C, Fischer-Betz R, Haase I, Broder A, Kaur N, Buyon J, Cohen B, Kamen D, English J, Arar A, Kim M. Predicting Adverse Pregnancy Outcomes in Women with Systemic Lupus Erythematosus: External Validation of the PROMISSE Model Using Multiple Independent Cohorts [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/predicting-adverse-pregnancy-outcomes-in-women-with-systemic-lupus-erythematosus-external-validation-of-the-promisse-model-using-multiple-independent-cohorts/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predicting-adverse-pregnancy-outcomes-in-women-with-systemic-lupus-erythematosus-external-validation-of-the-promisse-model-using-multiple-independent-cohorts/