Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Azathioprine is a thiopurine used to treat inflammatory conditions; however, it is often discontinued due to dose-dependent bone marrow toxicity. The Pharmacogenomics Knowledgebase (PharmGKB) lists 25 genes in the metabolic pathway, therefore, small effect changes caused by variants in these genes may combine to large effect. Only two of these genes (TPMT and NUDT15) are in clinical guidelines for thiopurine use and only explain less than 25% of azathioprine-induced bone marrow toxicity. We hypothesize that a risk score that combines the expression of other genes in the thiopurine metabolic pathway would be associated with azathioprine discontinuation due to bone marrow toxicity among TPMT and NUDT15 normal metabolizers.
Methods: In a retrospective cohort of patients with vasculitis, SLE, RA, or other inflammatory conditions (e.g., autoimmune hepatitis, IBD) we identified new users of azathioprine who were TPMT and NUDT15 normal metabolizers and collected information from their clinical records. The outcome was possible bone marrow toxicity—defined as discontinuing azathioprine due to any cytopenia. Genotyping was performed using the MEGAchip and, following imputation and quality control, we used PrediXcan (GTEx version 8 MASHR-based eQTL models) to estimate the genetically regulated expression of 13 genes involved in azathioprine metabolism (ADK, AOX1, GDA, GMPS, GSTA1, GSTM1, IMPDH1, ITPA, NME1, NME2, NT5C2, PPAT, RMM2), and 4 involved in azathioprine transport (ABCC4, SLC29A1, SLC29A1, and SCL29A2). As most drug metabolism occurs in the liver, we selected liver-specific estimations. We used Lasso regression that included all 17 predicted gene expressions and 5 principal components for model selection (i.e. relaxed Lasso). We built a score based upon the resulting coefficients of selected variables. Based on score quintiles, we used cox hazard models to test whether scores were associated with azathioprine discontinuation due to cytopenia among Whites and whether the same score showed similar trends in those who reported other races or race unknown.
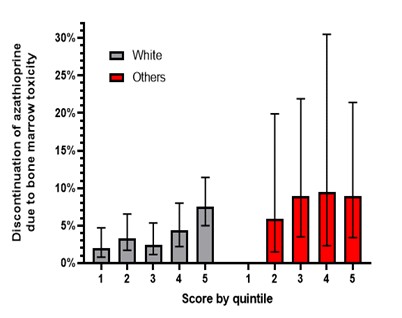
Results: We studied 1417 patients followed over a median of 1.9 years. Mean age was 43.6±17.6 years, 959 (67.7%) were female, initial daily dose 78.9± 47.8, and 1230 (87%) were White; 61 discontinued azathioprine due to cytopenia. The risk score included the predicted expression of the two genes (AOX1 and NME1) that had non-zero coefficients from the lasso regression. White patients with the highest score quintile had a significantly higher risk of discontinuing azathioprine due to cytopenia compared to those in the lowest quintile [HR=3.65 (95% C.I. 1.37-9.73, p=0.010]. Results remained significant after adjustment for age, sex, azathioprine indication, and azathioprine initial daily dose [HR=3.77 (95% C.I. 1.41-10.06), p=0.008]. A similar association was observed among 187 patients who either reported other races or race unknown (p< 0.001).
Conclusion: Among patients who were TPMT and NUDT15 normal metabolizers, a score combining AOX1 and NME1 expression was associated with discontinuation of azathioprine due to bone marrow toxicity. Further studies are needed to replicate these findings in different cohorts.
To cite this abstract in AMA style:
Daniel L, Dickson A, Zanussi J, Miller-Fleming T, Straub P, Wei W, Plummer D, Dupont W, Liu G, Anandi P, Reese T, Birdwell K, Kawai V, Hung A, Cox N, Feng Q, Stein C, Chung C. Predicted Expression of Genes Involved in the Thiopurine Metabolic Pathway Is Associated with Azathioprine Discontinuation Due to Bone Marrow Toxicity [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/predicted-expression-of-genes-involved-in-the-thiopurine-metabolic-pathway-is-associated-with-azathioprine-discontinuation-due-to-bone-marrow-toxicity/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predicted-expression-of-genes-involved-in-the-thiopurine-metabolic-pathway-is-associated-with-azathioprine-discontinuation-due-to-bone-marrow-toxicity/