Session Information
Date: Saturday, November 16, 2024
Title: B Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Autologous CD19-directed CAR-T cell therapy has been shown to eradicate aberrant B cells leading to durable clinical responses in SLE patients (Müller 2024; Wang 2024). However, autologous CAR-T cell therapy is characterized by logistical complexities of apheresis and long manufacturing times, which may be accompanied by extended periods of immunosuppressive washout. CB-010 is an allogeneic off-the-shelf CAR-T cell therapy derived from healthy donor T cells and engineered with a Cas9 CRISPR hybrid RNA-DNA (chRDNA) genome-editing technology. CB-010 features an anti-CD19 CAR derived from the FMC63 scFv and a 4-1BB costimulatory domain. Here we describe preclinical data supporting the recent FDA clearance of the CB-010 IND in both lupus nephritis (LN) and extrarenal lupus (ERL) patients in 1H 2024.
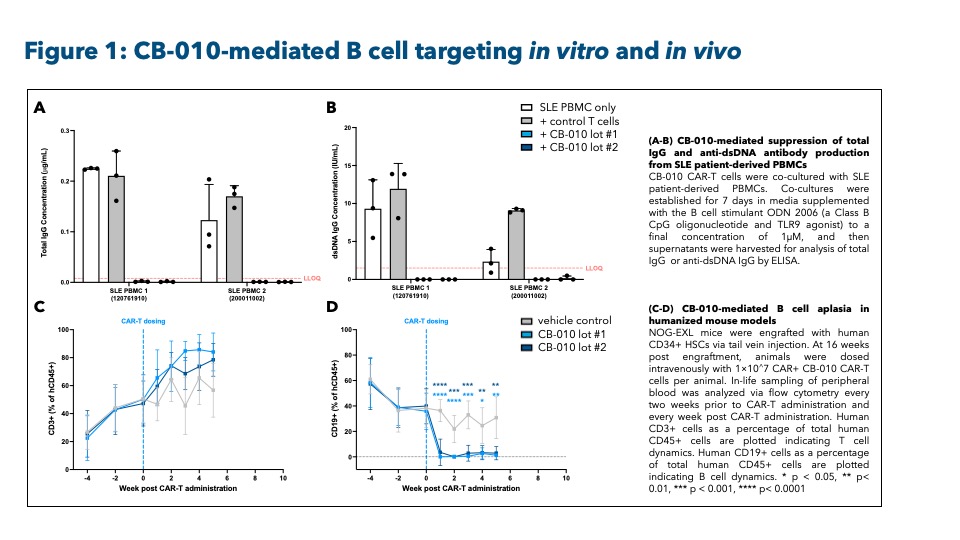
Methods: Cas9 chRDNA guides were implemented to generate 3 genome edits in the manufacture of CB-010: (1) knockout of the TRAC gene to eliminate T cell receptor expression, (2) site-specific insertion of a CD19-targeted CAR expression cassette into the TRAC locus, and (3) knockout of PDCD1, the gene encoding PD-1. In vitro preclinical studies were performed to evaluate CB-010-targeted B cell depletion and associated effects on IgG production and anti-double stranded DNA (dsDNA) production. Further, NOG-EXL mice were engrafted with human CD34+ cells to generate humanized mouse models to recapitulate the human peripheral hematopoietic compartment and examine B cell targeting following CB-010 CAR-T cell administration.
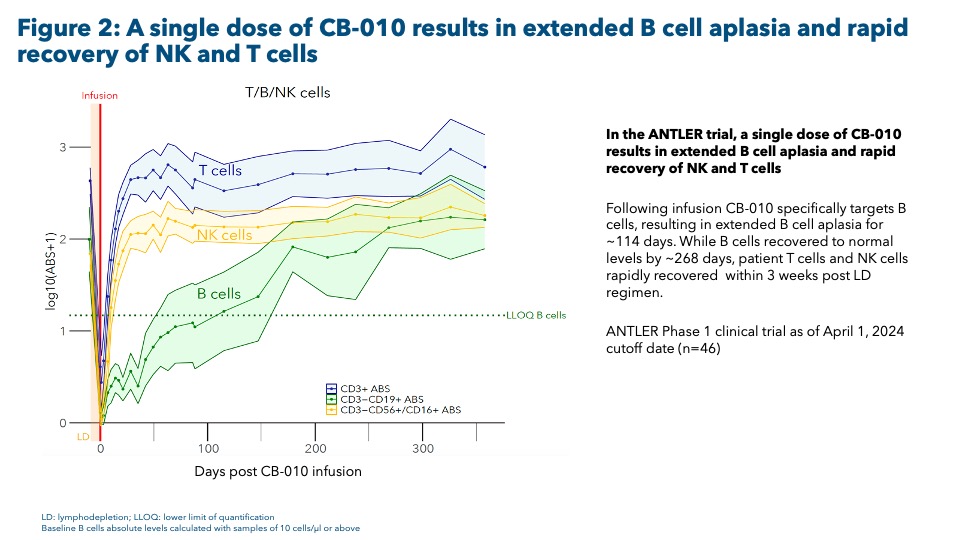
Results: Following in vitro stimulation of SLE patient-derived B cells, co-culture with CB-010 suppressed both IgG production and anti-dsDNA antibody production. In addition, B cell-targeted cytotoxicity was observed in co-cultures of CB-010 with SLE patient-derived peripheral blood mononuclear cells (PBMCs). In humanized mouse models, CB-010 led to targeted B cell aplasia without impacting other evaluated immune cell types (see Figure 1). Furthermore, in the ongoing ANTLER phase 1 clinical trial (NCT04637763) in relapsed/refractory B cell non-Hodgkin lymphoma patients, CB-010 activity is associated with prolonged B cell aplasia (see Figure 2). The duration of B cell aplasia observed with CB-010 was similar to the clinically relevant timing of CAR-T cell-mediated B cell depletion reported in SLE patients in recently published data (Müller 2024).
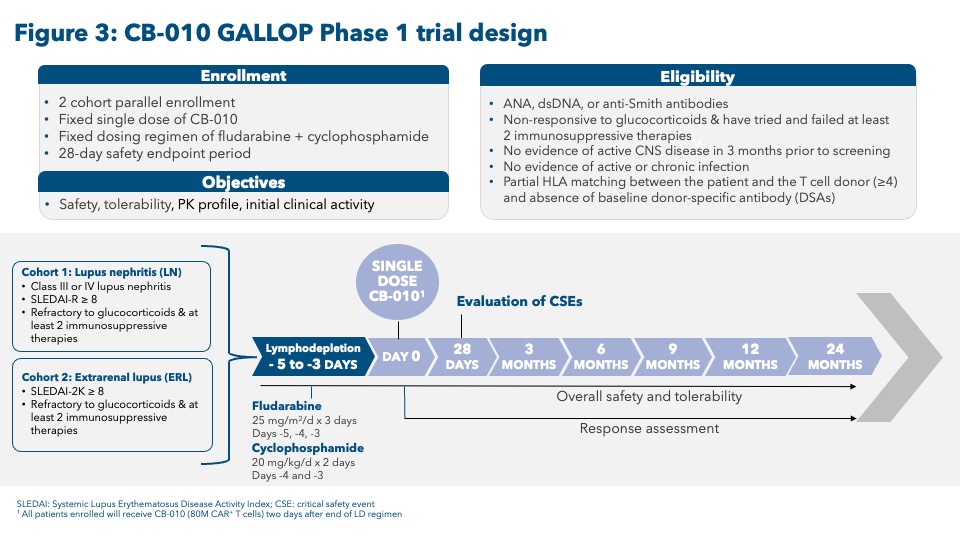
Conclusion: CB-010, an off-the-shelf CAR-T cell therapy, demonstrated specific B cell targeting in preclinical studies both in vitro and in vivo. The combination of these preclinical data, with a generally well tolerated clinical safety profile and encouraging efficacy from the ongoing ANTLER clinical trial, support the evaluation of CB-010 in a Phase 1 clinical trial for refractory LN and ERL patients (GALLOP). Two patient cohorts (one each in LN and ERL) will be enrolled in parallel using lymphodepletion with fludarabine and cyclophosphamide followed by a single CB-010 infusion of 80 x 106 CAR-T cells (see Figure 3).
To cite this abstract in AMA style:
Garner E, Kwong G, Fowler T, Kufeldt H, Kerfs B, Kim J, Aviles A, Alexander L, Davi F, Holland C, Maziere J, Garrett A, Bryan M, Chu L, Bhaduri S, Gerber M, Alambra E, Skoble J, Kochy T, Nesheiwat T, Portella S, Zudaire E, Kanner S. Preclinical Analysisof CB-010, an Allogeneic anti-CD19CAR-T Cell Therapywith a PD-1 Knockout, for the Treatment of Patients with Refractory Systemic Lupus Erythematosus (SLE) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/preclinical-analysisof-cb-010-an-allogeneic-anti-cd19car-t-cell-therapywith-a-pd-1-knockout-for-the-treatment-of-patients-with-refractory-systemic-lupus-erythematosus-sle/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preclinical-analysisof-cb-010-an-allogeneic-anti-cd19car-t-cell-therapywith-a-pd-1-knockout-for-the-treatment-of-patients-with-refractory-systemic-lupus-erythematosus-sle/