Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
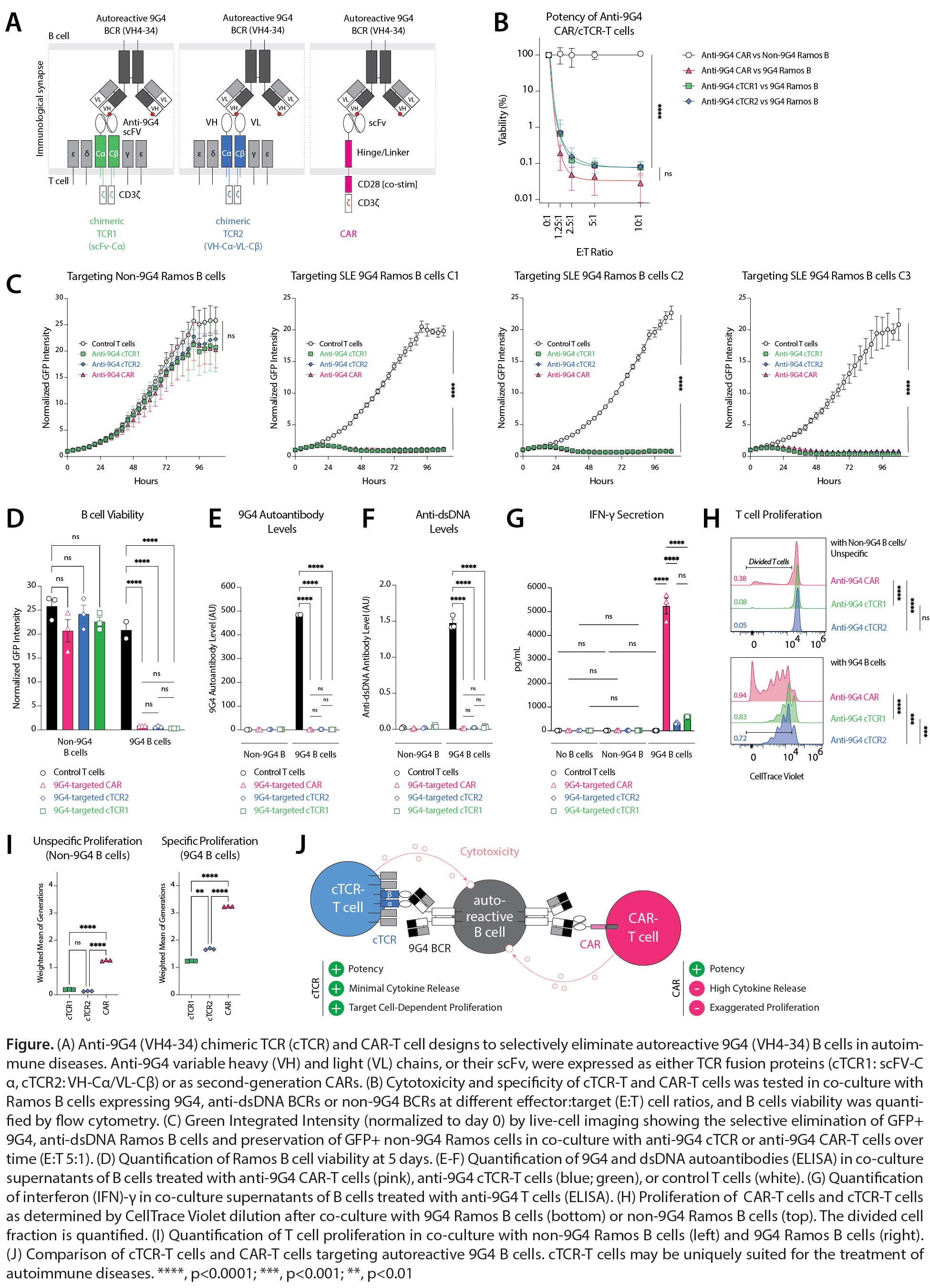
Background/Purpose: The autoreactive B cell compartment in systemic lupus erythematosus (SLE) is characterized by expansion of B cells expressing immunoglobulin heavy variable gene 4-34 (IGHV4-34) B cell receptors (BCRs). This innately autoreactive B-cell population carries the 9G4 idiotope (9G4) and is a major source of disease-relevant autoantibodies in lupus, cold agglutinin disease (CAD), and other autoimmune diseases. While CD19-CAR-T cells have transformed the treatment of refractory SLE, pan-B cell depleting therapies carry an increased risk of infection. By contrast, precision immunotherapies that deplete autoreactive B cells, while sparing other B cells, may treat SLE without causing immunosuppression. To achieve this, we developed synthetic immune receptor T-cell therapies to selectively eliminate 9G4 B cells in patients with SLE (A).
Methods: Ramos B cells were CRISPR-Cas9-edited to replace their endogenous BCR with monoclonal BCRs from patients with SLE (“SLE 9G4 Ramos C1-C3”), CAD, or APS. Homology-directed repair template, encoding an anti-9G4 CAR or anti-9G4 cTCR, and Cas12a-gRNA ribonucleoprotein targeting TCR genes were transfected into activated T cells purified from PBMCs of healthy donors and patients with SLE. CAR/cTCR expression was quantified by flow cytometry, and edited T cells were isolated by positive selection. The potency and specificity of engineered T cells against Ramos B cells were quantified by live-cell imaging, flow cytometry, CellTrace Violet dye dilution to measure T-cell proliferation, and interferon (IFN)-γ ELISA. SLE PBMCs were treated with engineered T cells, and depletion of IgG 9G4 B cells was visualized by anti-9G4 FluoroSpot. Autoantibody depletion was quantified using custom 9G4 and dsDNA ELISAs.
Results: Anti-9G4 CAR-T cells and anti-9G4 cTCR-T cells, integrating anti-9G4 antibody fragments into a re-engineered TCR (A), eliminated autoreactive SLE-9G4 Ramos B cells (B-D) and their secreted anti-dsDNA autoantibodies (E-F) with equal potency (p >0.99), while sparing non-9G4 Ramos B cells. Despite ~17-fold lower IFN-γ secretion compared to CAR-T cells (315 [SD 41] vs 5237 [SD 570] pg/mL for cTCR2 vs CAR, p< 0.0001), anti-9G4 cTCR-T cells achieved equal cytotoxicity in one-time and repeated B cell stimulation assays (G). Increased cytokine release by CAR-T cells was paralleled by greater T-cell proliferation following 9G4 B cell exposure (weighted mean of generations 3.24 vs 1.67 for CAR vs cTCR2, p< 0.0001). Notably, 9G4 B cell-independent proliferation was also observed in CAR-T cells, but not with cTCR-T cells (37.7% vs 5.1% T cells divided; weighted mean of generations 1.28 vs 0.14, p< 0.0001) (H-I). In co-culture with SLE PBMCs, donor-matched cTCR-T cells and CAR-T cells depleted primary human 9G4 B cells in a dose-dependent manner.
Conclusion: We describe precision cellular immunotherapies for the depletion of autoreactive, 9G4 B cells in SLE, CAD, and other autoimmune diseases. Both anti-9G4 CAR-T cells and anti-9G4 cTCR-T cells completely and efficiently eliminated primary or cancerous 9G4 B cells. cTCR-T cells, as compared to CAR-T cells, have preferable cytokine release/safety characteristics for the treatment of patients with autoimmune diseases (J).
To cite this abstract in AMA style:
Liu J, Mog B, Xia Y, Shaw E, Pearlman A, Ferris D, Kaeo K, Gliech C, Awosika T, Moritz B, Nichakawade T, Li Y, Glavaris S, DiNapoli S, Marcou N, Ahmedna T, Duarte Alvarado V, Wirtz D, Bugrovsky R, Jenks S, Sanz I, Goldman D, Petri M, Bettegowda C, Paul S, Kinzler K, Zhou S, Andrade F, Vogelstein B, Konig M. Precision Targeting of Autoreactive 9G4 B Cells in Systemic Lupus Erythematosus Using Engineered Chimeric Antigen Receptor (CAR)- and Chimeric T Cell Receptor (cTCR)-T Cells [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/precision-targeting-of-autoreactive-9g4-b-cells-in-systemic-lupus-erythematosus-using-engineered-chimeric-antigen-receptor-car-and-chimeric-t-cell-receptor-ctcr-t-cells/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/precision-targeting-of-autoreactive-9g4-b-cells-in-systemic-lupus-erythematosus-using-engineered-chimeric-antigen-receptor-car-and-chimeric-t-cell-receptor-ctcr-t-cells/