Session Information
Date: Saturday, November 16, 2024
Title: B Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: With exponential demand for chimeric antigen receptor (CAR) therapies for autoimmune disease, allogeneic options will be essential. Advantages include use of healthy donor cells, ready availability, reduced cost, and the ability to continue to treat patients with immunosuppressive therapies through infusion, if compatible with CAR-T function. However, several limitations will need to be overcome. Amongst these are that off-the-shelf CAR-T cells will need strategies to help evade recipient-mediated rejection, as well as strategies to enable their survival and in vivo expansion in patients receiving immunomodulating therapies. One strategy to address both issues would be to create CAR-T cells resistant to established immunosuppressive therapies. The calcineurin inhibitors (CNIs) cyclosporine (CsA) and voclosporin (VCS) are two such drugs. Both CsA and VCS only interact with calcineurin when associated with a partner protein, called cyclophilin A (CypA). Here we created CAR-T cells resistant to both CsA and VCS with a single CRISPR-based gene edit of the cyclophilin A gene (PPIA) to create a CypA protein that disrupts the ability of these drugs to inhibit calcineurin.
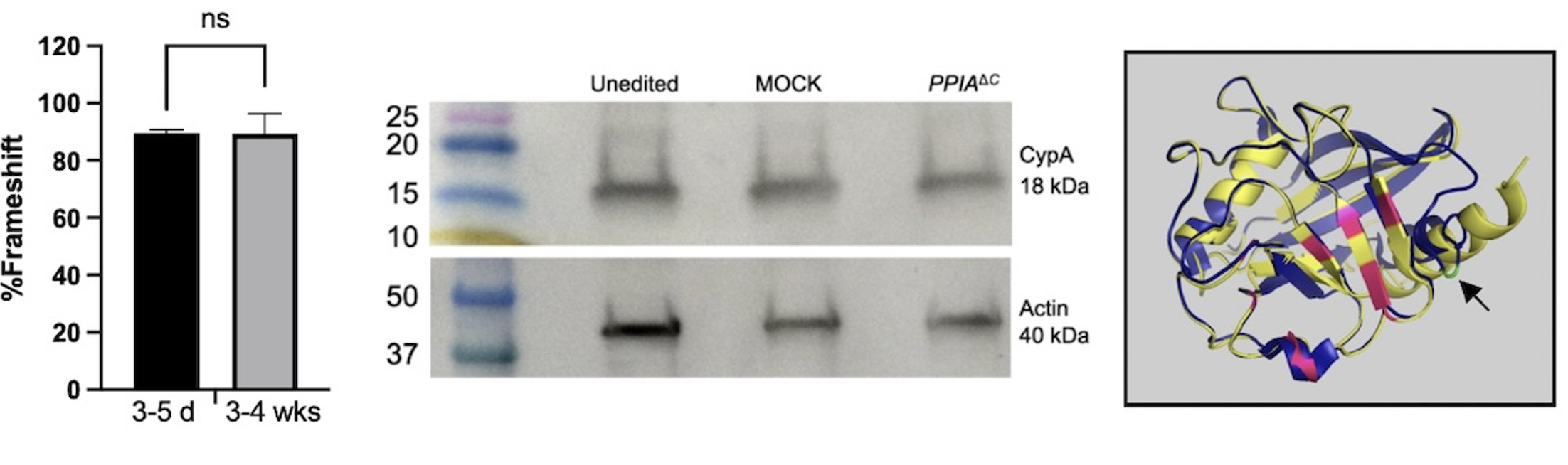
Methods: We engineered CsA/VCS resistance by CRISPR-Cas9 editing of PPIA. As CypA is a highly expressed protein with many homeostatic functions in T cells, we focused on targeted disruption of the terminal exon of PPIA to avoid non-sense mediated decay and resultant complete knockout. This resulted in a protein that has high structural homology to wild-type CypA in its catalytic site, but diverges at the C-terminus where important residues are located for interaction with calcineurin A (edited protein denoted as PPIAΔC).
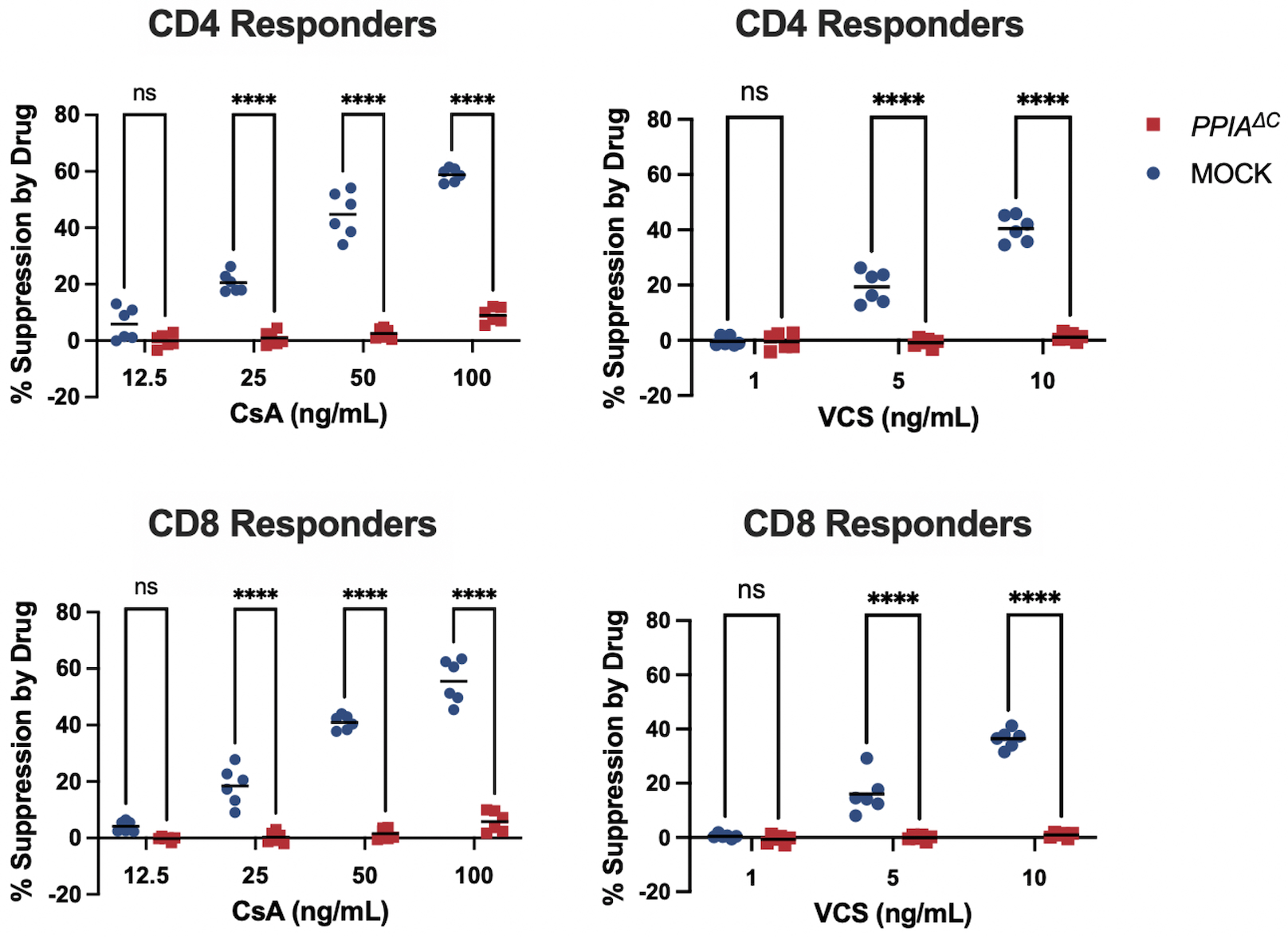
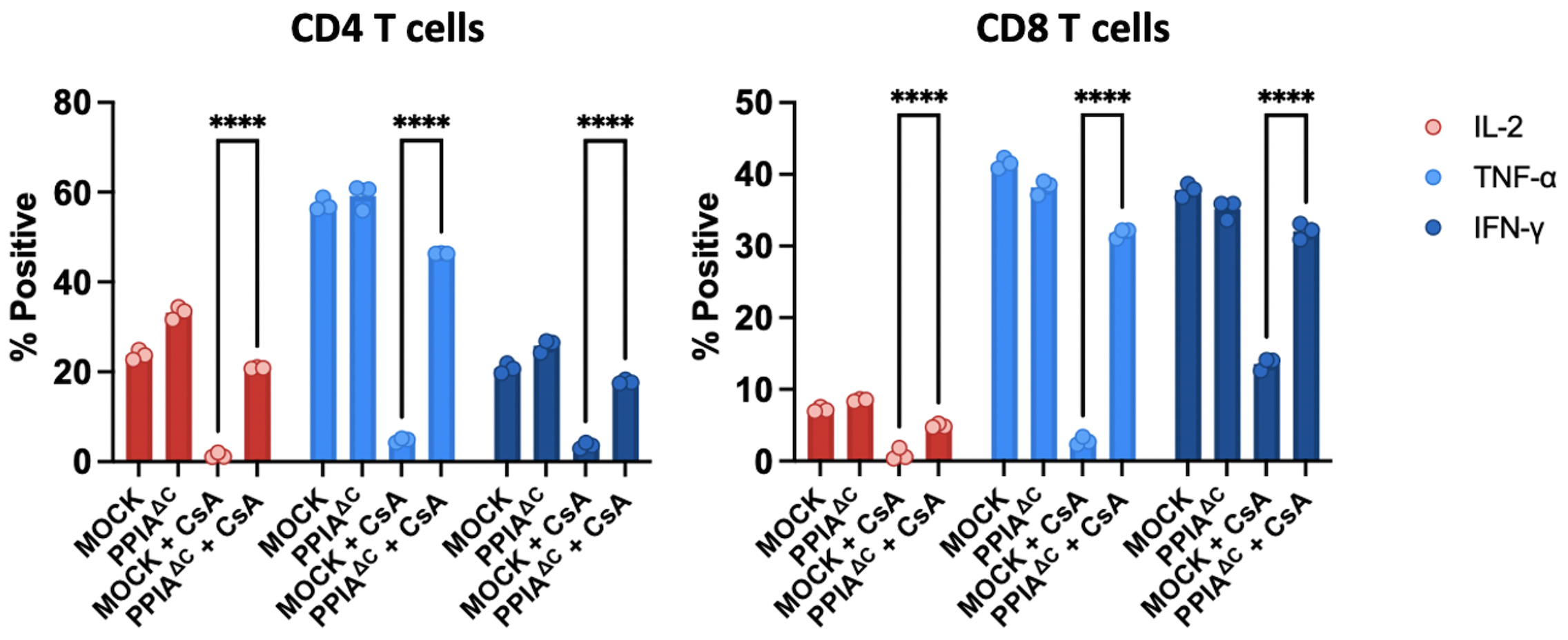
Results: We verified that the C-terminal editing strategy resulted in a highly reproducible indel profile that was stable over 4 weeks in culture, and that PPIAΔC T cells retained CypA expression (Figure 1). To demonstrate drug resistance, we introduced the PPIAΔC edit or a MOCK edit into CD19-CAR-T cells. As expected, when co-cultured with the CD19+ NALM6 cell line, proliferation of MOCK-edited CAR-T cells was readily suppressed by CsA and VCS in a dose dependent manner. Importantly, PPIAΔC edited CAR-T cells were resistant to CsA and VCS mediated suppression (Figure 2). For example, at a CsA dose of 50 ng/mL, there was 44.8 ± 7.9 % suppression of division of CD4+ MOCK edited CAR-T cells vs. 2.5 ± 1.9 % suppression of PPIAΔC cells (p < 0.0001). PPIAΔC CAR-T cells also significantly preserved CAR-mediated proinflammatory cytokine production in the presence of CsA relative to MOCK-edited cells, further substantiating their functional integrity despite drug exposure (Figure 3).
Conclusion: We edited PPIA to produce CAR-T cells resistant to CsA and VCS. This genetic modification may enable allogeneic CAR-T cells to be compatible with CNIs, a benefit for patients such as those with lupus nephritis being treated with CNIs, and as part of a strategy to help evade allogeneic CAR-T cell rejection. Engineering allogeneic products to function in the setting of different background immunomodulating therapies has the potential to expand the clinical scenarios in which CAR-T cells can be used in patients with autoimmune disease.
To cite this abstract in AMA style:
Wobma H, Alvarez-Calderon F, Dong J, Albanese A, Omdahl K, Bermea R, Selig G, Winschel M, Rojas Palato E, Michaelis K, Rui X, Blazar B, Prockop S, Tkachev V, Gerdemann U, Kean L. Precision Editing of Cyclophilin a to Engineer Cyclosporine- and Voclosporin- Resistant Human CAR-T Cells [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/precision-editing-of-cyclophilin-a-to-engineer-cyclosporine-and-voclosporin-resistant-human-car-t-cells/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/precision-editing-of-cyclophilin-a-to-engineer-cyclosporine-and-voclosporin-resistant-human-car-t-cells/