Session Information
Session Type: Abstract Session
Session Time: 10:30AM-11:30AM
Background/Purpose: For patients with pre-existing RA, immune checkpoint inhibitors (ICIs) for cancer treatment may trigger RA flares or other immune-related adverse events. Underlying altered immunity and need for immunosuppression in cancer patients with RA may also blunt the effectiveness of ICIs, leading to cancer progression and mortality. However, most previous studies investigated cancer patients with any pre-existing autoimmune condition initiating an ICI, rather than a specific condition, and lacked a comparator group without autoimmune disease. We aimed to investigate risk for RA flare and mortality for pre-existing RA cases and matched comparators who did not have RA initiating ICI for cancer treatment.
Methods: We performed a retrospective cohort study of patients initiating an ICI at large tertiary academic health system and cancer center (2011-2021). We used electronic query to identify all cancer patients who initiated an ICI. We then screened for RA among those with any diagnosis code for inflammatory arthritis prior to date of ICI initiation (index date). We confirmed pre-existing RA that met 2010 ACR/EULAR criteria through medical record review. We matched each RA case to up to 3 comparators at index date by age, sex, calendar year, specific histologic cancer type (verified by medical record review), and ICI target. The pool of eligible comparators had no diagnosis codes for any systemic rheumatic disease before index date. The outcomes of interest were RA flare and mortality, each obtained from medical record review. We obtained baseline characteristics on demographics, lifestyle, cancer, and RA using medical record review or administrative data. We described rates of RA flare and mortality among RA cases. For risk of RA flare, we handled the competing risk of death using Fine and Gray regression models. We compared mortality of pre-existing RA cases vs. matched comparators using Cox regression.
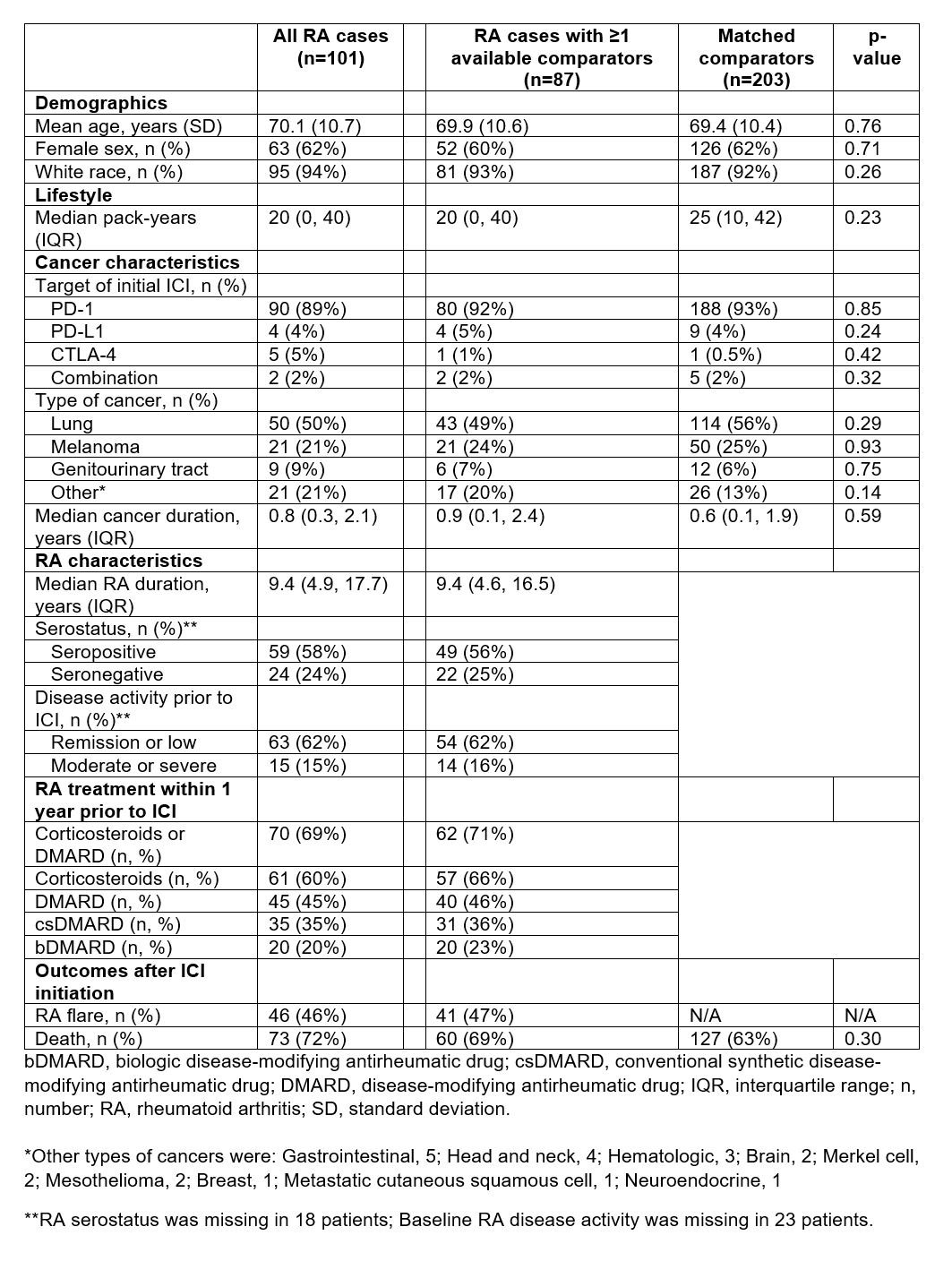
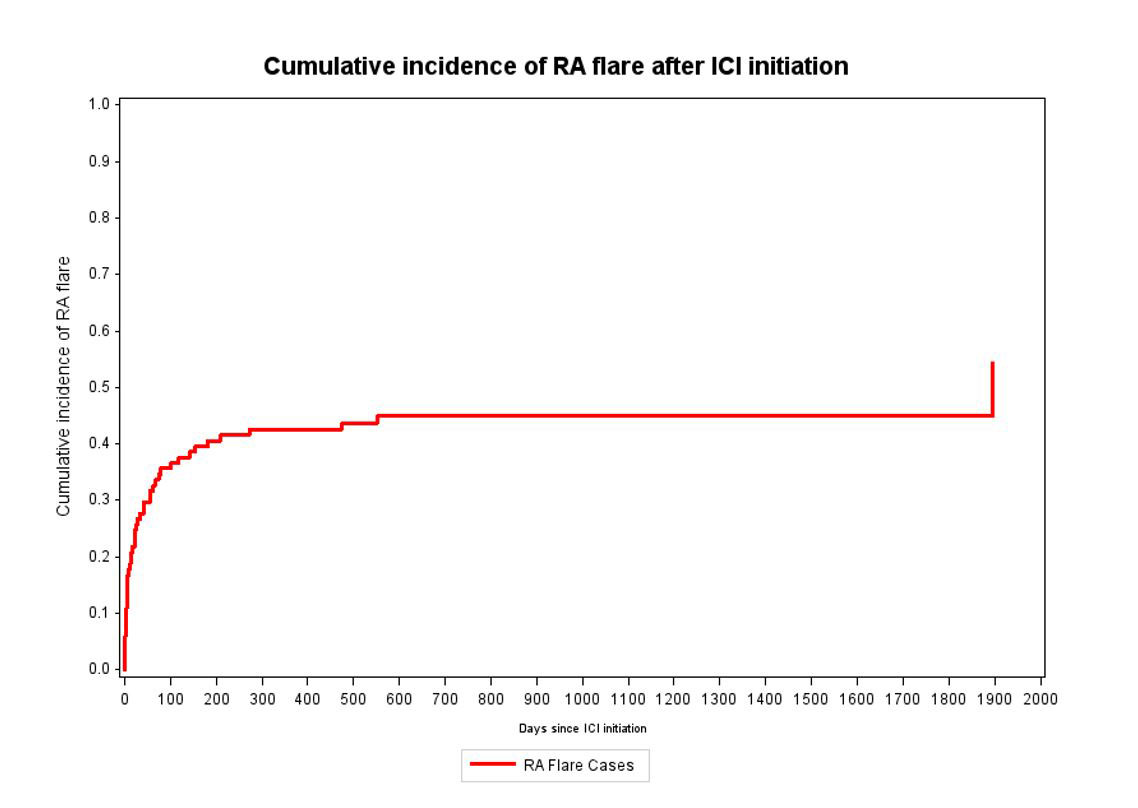
Results: Among 13,028 cancer patients initiating ICI, we identified 101 patients with pre-existing RA (mean age 70.1 years, 62% female, Table). Among RA cases, 46% had RA flares after initiating ICI (27% of all patients flaring within the first 28 days, see Figure 1) and 72% died during median 79 (IQR 21, 346) days of follow-up. Seropositivity was associated with increased risk of flare compared to seronegativity, even accounting for competing risk of death (multivariable subdistribution hazard ratio [sdHR] 1.95, 1.03-3.69). Baseline treatment of RA with corticosteroids/DMARDs was associated with sdHR of 1.89 (0.99-3.60) for post-ICI flare compared to no baseline treatment for RA. We were able to match 87 RA cases to 203 comparators. Among these RA cases and their comparators, 69% and 63% died, respectively. Pre-existing RA vs. comparators had a HR for total mortality of 1.20 (0.89-1.63, Figure 2).
Conclusion: Patients with pre-existing RA initiating ICI for cancer treatment had nearly 50% risk of RA flare, and 27% of all patients flared within the first month after ICI initiation. RA had similar mortality as matched comparators, suggesting that RA or its treatment may not have a large impact on cancer progression and mortality. These results argue that RA should not be considered a contraindication for appropriate ICI treatment.
To cite this abstract in AMA style:
McCarter K, Wolfgang T, Arabelovic S, Wang X, Yoshida K, Banasiak E, Qian G, Kowalski E, Vanni K, LeBoeuf N, Buchbinder E, Gedmintas L, MacFarlane L, Rao D, Shadick N, Gravallese E, Sparks J. Pre-existing Rheumatoid Arthritis Patients Initiating Immune Checkpoint Inhibitors for Cancer Treatment: A Comparative Cohort Study Investigating RA Flare and Mortality [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/pre-existing-rheumatoid-arthritis-patients-initiating-immune-checkpoint-inhibitors-for-cancer-treatment-a-comparative-cohort-study-investigating-ra-flare-and-mortality/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pre-existing-rheumatoid-arthritis-patients-initiating-immune-checkpoint-inhibitors-for-cancer-treatment-a-comparative-cohort-study-investigating-ra-flare-and-mortality/