Session Information
Date: Wednesday, October 24, 2018
Title: 6W020 ACR Abstract: Metabolic & Crystal Arthropathies–Basic Science & Imaging (2964–2969)
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
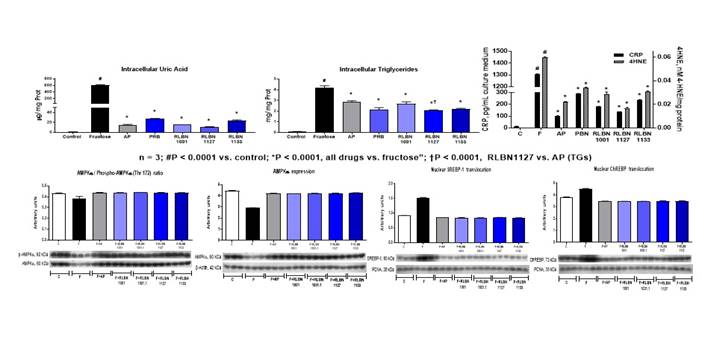
Background/Purpose: Dietary fructose promotes an increase in uric acid (UA) that may lead to gout. UA itself promotes lipogenesis and inflammation in both gout as well as other diseases, such as atherosclerosis, metabolic syndrome and NASH. In vivo, these effects can be blocked by hypouricemic drugs, but only in models with elevated UA. In vitro incubation with fructose also models lipogenic and inflammatory activities in hepatocytes. We discovered a prototype drug that markedly reduced serum UA in human subjects (frequently to < 1.0 mg/dL), and we developed potent bifunctional derivatives that inhibit xanthine oxidase (XO) and URAT1 (and uricase). We evaluated fructose-induced increases in intracellular (IC) UA and triglycerides in HepG2 cells, explored mechanisms and metabolic consequences, and their modulation by both mono- and bi-functional hypouricemic drugs.
Methods: Control HepG2 cells were cultured in DMEM w/low glucose w/o phenol red for 48-72 h and changed every 24 h. Test cells were cultured with added fructose (25mM), plus new or standard drugs (RLBN1001, RLBN11127, RLBN1133, allopurinol [AP], and probenecid [PBN]) (100mM). IC and extracellular (EC) UA and triglycerides (TGs) were measured by enzymatic kits (Sekisui). Lipid content was confirmed by Oil Red O and colorimetry (Cayman). Micro-CRP and 4-hydroynonenal (4HNE) were assessed by ELISA and colorimetry, respectively (Cayman). Experiments were conducted three times, each in triplicate. Expression of prolipogenic enzymes and inflammatory mediators were evaluated by WB.
Results: Fructose sharply increased IC UA, which was significantly suppressed by AP and RLBN1127 (95% and > 98%, respectively; see figure). Increased UA was accompanied by an increase in IC TGs with confirmed steatosis at 48 hours. While all hypouricemic drugs reduced IC TGs, RLBN1127 was significantly superior to AP in blocking TG production (P < 0.0001). All drugs reduced over-expression of fatty acid synthase, acetyl CoA carboxylase and ATP citrate lyase (data not shown). All drugs normalized fructose-related increases in nuclear translocation of SREBP-1 and ChREBP, and significantly reduced increases in 4HNE and CRP (graphic). Lastly, these drugs significantly increased and restored fructose-induced reductions in total expression and activity of AMP Kinase.
Conclusion: Suppression of fructose-induced increases in UA with hypouricemic drugs significantly reduced TGs and markers of lipid peroxidation and inflammation in hepatocytes. These agents acted via AMPK activation, a constitutive in vivo suppressor of lipogenesis and inflammation, which limits urate crystal-induced NF-kB activation and IL-1b release. These results suggest that bifunctional activity may confer advantages over monofunctional drugs. RLBN1127 is a clinical candidate for treatment of biomarker-targeted patients with inflammatory disease, particularly gout and NASH.
To cite this abstract in AMA style:
Sánchez Lozada LG, García-Arroyo FE, Juárez-Rojas JG, Gonzaga G, Warrell RP Jr.. Potent Bifunctional Inhibitors of Xanthine Oxidase and URAT1 Block Fructose-Induced Inflammation Via Increase in AMP Kinase Activity [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/potent-bifunctional-inhibitors-of-xanthine-oxidase-and-urat1-block-fructose-induced-inflammation-via-increase-in-amp-kinase-activity/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/potent-bifunctional-inhibitors-of-xanthine-oxidase-and-urat1-block-fructose-induced-inflammation-via-increase-in-amp-kinase-activity/