Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
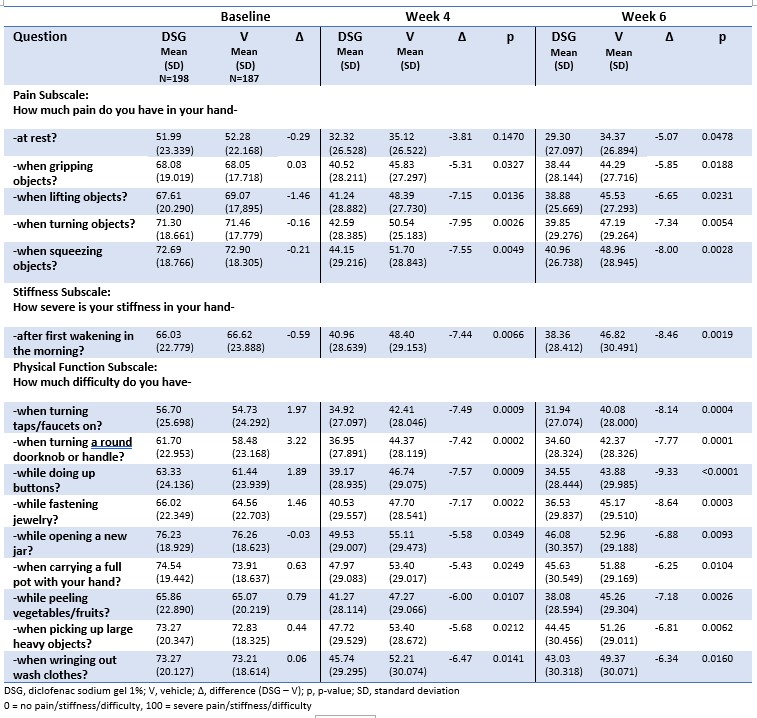
Background/Purpose: Hand osteoarthritis (OA) is a frequently occurring form of OA characterized by symptoms including joint pain, stiffness, and swelling which affect performance of activities of daily living.The Australian/Canadian Osteoarthritis Hand Index (AUSCAN) is a key functional survey for hand OA, consisting of 15 questions organized into three subscales: pain, stiffness, and physical function.While AUSCAN results are typically reported as a total index and/or subscale scores, the individual questions are more granular endpoints which are relevant to patients’ daily activities such as getting dressed, turning doorknobs and faucets, and cooking. The purpose of this post-hoc analysis was to evaluate the scores from the individual AUSCAN questions from an 8-week randomized, double-blind, placebo-controlled trial of diclofenac sodium 1% gel (DSG) vs vehicle in the treatment of hand OA (Altman RD, et al. J Rheumatol 2009;36:1991-9).
Methods: Patients diagnosed with hand OA in the dominant hand were randomly assigned DSG (n = 198) or vehicle (n = 187) and applied 2 g to each hand 4 times daily for 8 weeks. Vehicle was identical to DSG except for the absence of diclofenac sodium.AUSCAN questions were scored using a scale of 0 (no pain/stiffness/difficulty)-100 (extreme pain/stiffness/difficulty) at baseline and at weeks 1, 2, 4, 6 and 8.Mean scores for each question were assessed and compared between treatments at each timepoint using Analysis of Covariance (ANCOVA) with baseline as a covariate in the model and with no multiplicity correction.
Results: DSG demonstrated efficacy with reduction of pain subscale question scores at week 6 by 42%-44% (33%-35% for vehicle), stiffness scores by 42% (30% for vehicle) and physical function subscale scores by 39%-45% (27%-33% for vehicle).Statistically significant differences favoring DSG over vehicle were observed at weeks 4 and 6 (the primary endpoints) in all categories and at week 8 in the stiffness and physical function questions. Specifically, the questions with the greatest separation from vehicle at week 6 involved pain when turning and squeezing objects, stiffness upon wakening, difficulty turning doorknobs and faucets, and difficulty getting dressed, all of which are important components of hand functionality in daily living. Questions that did not reach statistical significance demonstrated a trend favoring DSG.
Conclusion: The results from this analysis show that the individual AUSCAN index question scores for DSG reach statistical significance vs vehicle at week 4 and last through week 8.The DSG scores at week 6 demonstrate a 39%-45% improvement from baseline in performing daily activities including turning objects such as doorknobs and faucets, getting dressed, and cooking compared to 27%-35% reductions from baseline for vehicle. These endpoints may be more meaningful to patients than the subscale scores of pain, stiffness and physical function, and may provide healthcare providers better ways to communicate the benefit of using DSG to treat the symptoms of hand OA.
To cite this abstract in AMA style:
Nicholson K, Sanchez E, Wright W, Block J, Petruschke R. Post-hoc Analysis of Individual Questions from the Australian/Canadian Osteoarthritis Hand Index from a Randomized, Double-blind, Placebo-controlled Trial of Patients with Hand OA [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/post-hoc-analysis-of-individual-questions-from-the-australian-canadian-osteoarthritis-hand-index-from-a-randomized-double-blind-placebo-controlled-trial-of-patients-with-hand-oa/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/post-hoc-analysis-of-individual-questions-from-the-australian-canadian-osteoarthritis-hand-index-from-a-randomized-double-blind-placebo-controlled-trial-of-patients-with-hand-oa/