Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Autoimmunity after COVID-19 infection has been reported. We examined connective tissue disease (CTD) symptoms and autoantibodies, SARS-CoV-2 serologies, and T and B cell immunophenotypes (found in rheumatoid arthritis, systemic lupus erythematosus, and scleroderma in past studies), by the early vs. late COVID-19 pandemic waves. We hypothesized that patients who had COVID in earlier (3/1/2020- 1/31/2021) vs. later (2/1/2021-9/1/2021) waves would have higher prevalence of new CTD symptoms, autoantibodies, and abnormal immunophenotypes.
Methods: We identified patients COVID-19 PCR positive from 3/1/2020-9/1/2021 in the Mass General Brigham system. Eligible participants ≥18 years old and ≥1-month post-COVID-19 with no prior history of CTD received the CTD Screening Questionnaire (CSQ; Karlson EW, 1995) to complete. Subjects who returned questionnaires were invited for a blood draw. Subjects were tested for 27 autoimmune and 3 SARS-CoV-2 serologies, and for T peripheral helper cells (TpH), T follicular helper cells (TfH), age-related B cells (ABCs), and plasmablasts (PBs) proportions by flow cytometry. We tested for associations between clinical variables, serologies, and immunophenotypes (% total CD4 T cells for TpH and TfH; % total CB19 B cells for ABC and PB) using t-tests, Chi-square, and multivariable logistic regression, adjusting for age, race, sex. A heatmap of the multidimensional data was generated to elucidate underlying patterns.
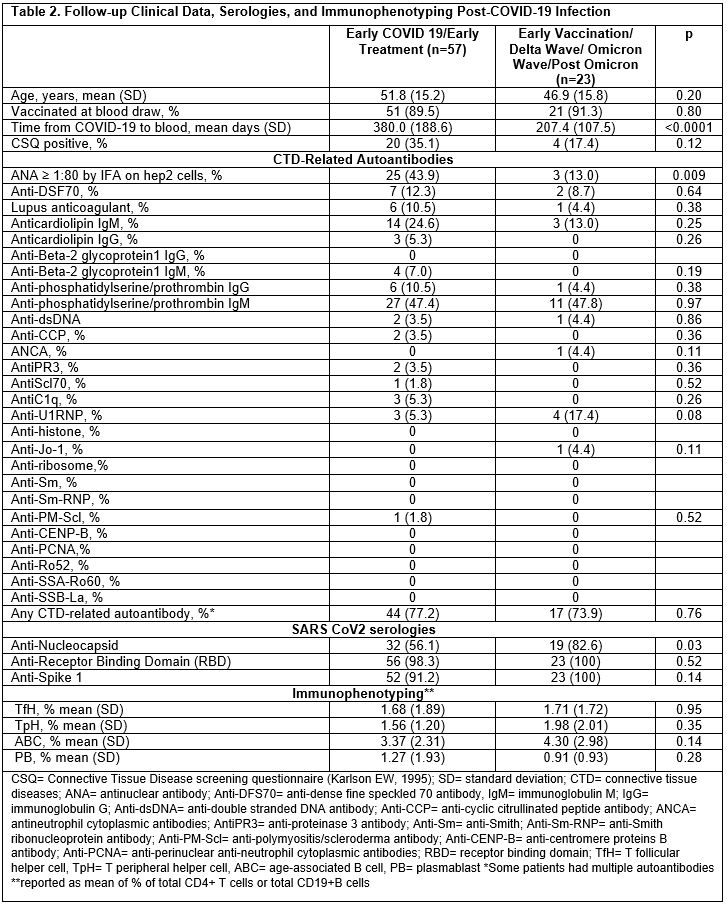
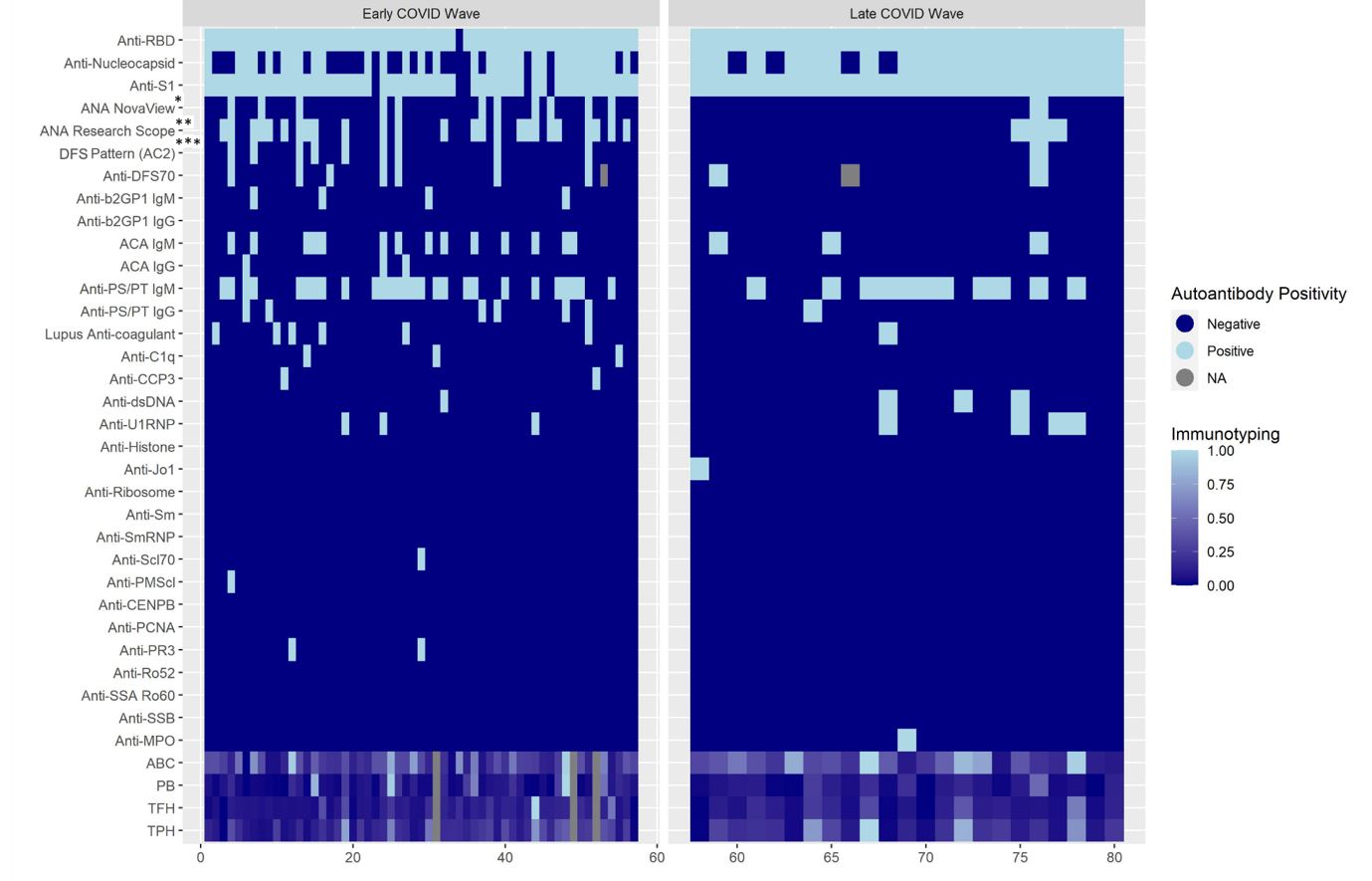
Results: 324 of 2,935 screening questionnaires (11%) were returned; 80 eligible subjects participated. Baseline demographics and clinical data are shown in Table 1 by early vs. late COVID-19 wave. 17 subjects were vaccinated at infection, all in the later pandemic; 10 subjects were hospitalized for COVID-19. Among those in earlier vs. later COVID waves, a higher proportion were CSQ+ (35% vs. 17%, p 0.12), and more were ANA+ (44% vs. 13%, p 0.009) (Table 2). Lupus anticoagulant was positive in 11% of early vs. 4% of late COVID, p 0.38. Having ≥1 positive autoantibody was found in 77% of early vs. 74% of later COVID subjects, p 0.76. After adjustment for age, race, and sex, having early (vs. late) COVID variants was associated with increased risk of ANA positivity (MV OR 4.55, 95%CI 1.16, 16.67). The heatmap revealed more variety of autoantibody positivity in early COVID, with more antibodies to cardiolipin IgG, B2GP1 IgM, C1q, CCP3, PMScl, PR3, Scl70, while Jo-1 and MPO were only present in later COVID waves. No clear patterns were seen in T and B cell phenotypes (Figure 1).
Conclusion: Although a small study without controls, infection in earlier vs. later COVID was associated with more ANA positivity and autoimmune rheumatic disease symptoms (latter non-significantly). Heatmap analyses elucidated increased prevalence of other autoantibody positivity in the earlier wave. These results raise the possibility that more virulent SARS-CoV-2 strains circulating in the early pandemic and pre-vaccine availability were more immunogenic and more likely to result in autoantibody production.
To cite this abstract in AMA style:
Oakes E, Buhler K, Adejoorin I, Marks K, Dillon E, Ellrodt J, Yee J, Rao D, Choi M, Costenbader K. Post-COVID-19 Autoimmune Serologies and Immunophenotypes [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/post-covid-19-autoimmune-serologies-and-immunophenotypes/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/post-covid-19-autoimmune-serologies-and-immunophenotypes/