Session Information
Session Type: Abstract Session
Session Time: 5:00PM-6:00PM
Background/Purpose: In patients with active systemic lupus erythematosus (SLE), circulating platelets have an activated phenotype characterized by the expression of P-selectin (CD62P). We have shown that in human SLE, platelets interact with T regulatory cells and repress their immunosuppressive functions through a P-selectin/CD15s-dependent interaction (1). We sought to investigate for other platelet/immune cell interaction and their potential relevance in SLE.
Methods: Patients with SLE responding to the 2019 ACR/EULAR criteria were recruited for blood sampling (n = 30). The expression of CD15s (P-selectin ligand) was assessed on all circulating immune cell subset using flow cytometry. Platelet-neutrophils aggregates were identified as (platelet) CD61+ (neutrophil) CD66b+ cells using flow cytometry on fresh blood samples. Single-cell cytosolic calcium and ROS imaging was performed by incubating cell with either a fluorescent calcium dye (cali-520), or a mitochondrial specific dye (MitoSox). Platelet-free plasma was isolated by two sequential centrifugations (3500xg) of EDTA-anticoagulated blood and stored for subsequent evaluation of soluble P-selectin (using ELISA) and (platelet-derived) microparticular P-selectin using flow cytometry.
Results:
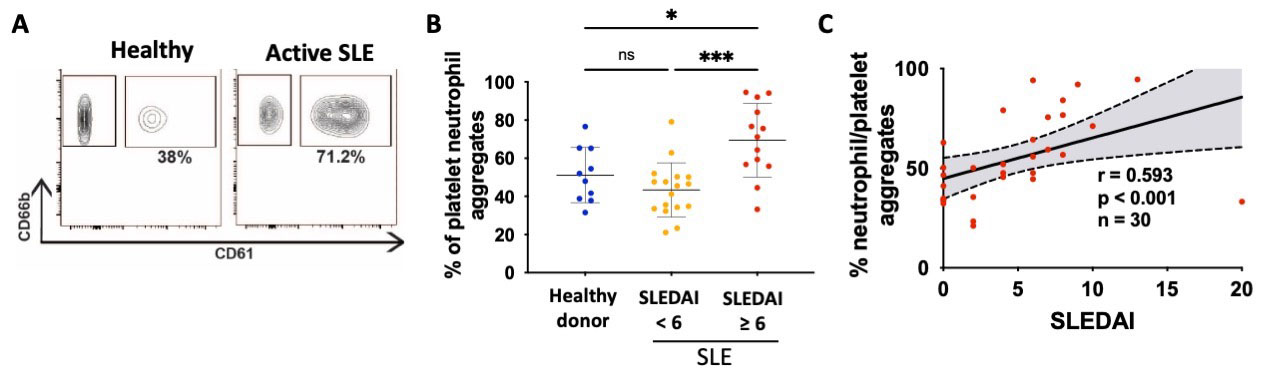
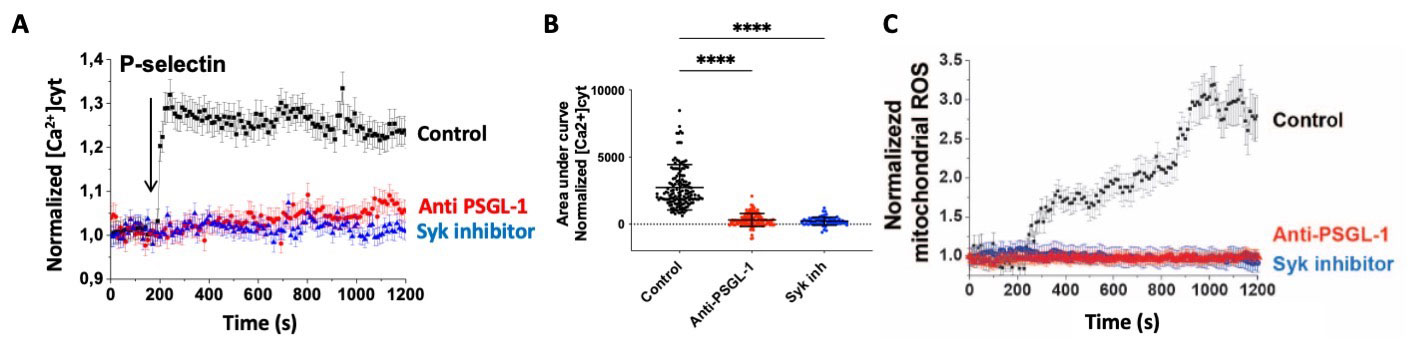
Results: In healthy donors (HD) and patients with SLE, circulating neutrophils expressed significantly higher levels of the P-selectin ligand CD15s compared to other immune subsets (p < 0.001), predicting platelet/neutrophil interactions. In contrast to HD and patients with inactive SLE, patients with active SLE had significantly more circulating platelet-neutrophils aggregates (p < 0.001; Fig. 1A-B), and these aggregates correlated with the SLEDAI (r = 0.59, p < 0.001; Fig. 1C). The incubation of human neutrophils with recombinant P-selectin induced a strong intracellular calcium signaling which was inhibited by an anti-PSGL1 antibody (blocking P-selectin/CD15s interaction) or with a Syk kinase inhibitor (Fig. 2A-B). Similarly, P-selectin induced a mitochondrial ROS release in a CD15s- and Syk-dependent manner (Fig. 2C). Interestingly, incubation of neutrophils with anti-dsDNA IgG and P-selectin induced mitochondrial depolarization, which was absent with either stimulus alone. Soluble and platelet-derived microparticular P-selectin levels were significantly increased in patients with active SLE compared to inactive patients or healthy donors (p < 0.05 and p < 0.001, respectively). In a longitudinal analysis of SLE patients, soluble and microparticular P-selectin levels closely followed clinical (SLEDAI) and biological (C3 levels) markers of SLE disease activity.
Conclusion: P-selectin levels are increased in active SLE and follow hallmarks of the disease activity. P-selectin induces calcium/mitochondrial ROS signaling in lupus neutrophils which are key players in SLE pathogenesis. We hypothesize that that platelet-neutrophil interaction participate in SLE pathogenesis and that blocking these interaction may represent a target in SLE.
Reference:
1) Scherlinger M. et al. (2021), Science Translational Medicine, 13(600):eabi4994.
Fresh blood was drawn from healthy donors and SLE patients (n = 30), and flow cytometry was conducted. (A) Gating strategy for the platelet/neutrophils aggregates (gated on CD66b+ cells). (B) Cumulative results for platelet/neutrophil aggregates. (C) Spearman correlation between platelet/neutrophil aggregates and the SLE disease activity index (SLEDAI). Each point represents one patient and bars indicate S.D. *, p < 0.05; ***, p < 0.001 using one-way ANOVA with Sidak's correction.
(A) Single-cell relative fluorescence using Cali-520 reflecting intracytoplasmic calcium concentration in healthy donors neutrophils stimulated with P-selectin (100ng/mL), with or without pre-incubation with anti-PSGL1 antibody or a Syk inhibitor. (B) Cumulative data of (A) experiment, each point represents one cell and bars indicate S.D; ****, p<0.001 using one-way ANOVA with Sidak's correction. (C) Single-cell MitoSox fluorescence representing intracellular mitochondrial reactive oxygen species in healthy donors neutrophils stimulated with P-selectin (100ng/mL), with or without pre-incubation with anti-PSGL1 antibody or a Syk inhibitor.
To cite this abstract in AMA style:
Scherlinger M, Vacher P, Guillotin V, Douchet I, Richez C, Blanco P. Platelet-selectin Prime Lupus Neutrophils to Produce Mitochondrial ROS and Participate in SLE Pathogenesis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/platelet-selectin-prime-lupus-neutrophils-to-produce-mitochondrial-ros-and-participate-in-sle-pathogenesis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/platelet-selectin-prime-lupus-neutrophils-to-produce-mitochondrial-ros-and-participate-in-sle-pathogenesis/