Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Antiphospholipid syndrome (APS) is an autoimmune disease with thrombotic and obstetric complications arising via a model of immunothrombosis. Patients may present with a spectrum of phenotypes, including thrombotic (tAPS), obstetric (oAPS), or catastrophic/microvascular APS (C/MAPS), while others may have antiphospholipid antibodies (aPL) without disease manifestations. Mechanisms underlying the development of these diverse phenotypes remain uncertain. Proteomic profiling was used in other thrombotic and microvascular disorders to highlight potential mechanisms of disease pathogenesis and may have a role in understanding the pathophysiology of APS.
We performed multiplex plasma proteomic profiling in aPL-positive patients with different clinical phenotypes to gain a greater understanding of potential immunothrombotic mechanisms in the pathogenesis of APS.
Methods: A web-based data capturing system was used to store patient demographics, history, and medications. The inclusion criteria were positive aPL per Updated Sapporo Classification Criteria tested within 1 year prior to enrollment. Multiplex proteomic profiling measuring approximately 7,000 unique proteins (SomaLogic; Boulder, CO, USA) was performed on 40 primary aPL-positive patient plasma samples from APS ACTION Registry (10 each of tAPS with/without oAPS, oAPS only, C/MAPS, and positive aPL without APS classification), and 10 of healthy controls. Differentially abundant proteins among all phenotypic groups and in pairwise comparisons were determined by applying ANOVA and t-tests to log-normalized data, respectively, with p< 0.05 considered statistically significant. Tests were adjusted for false discovery to minimize the likelihood of false positives.
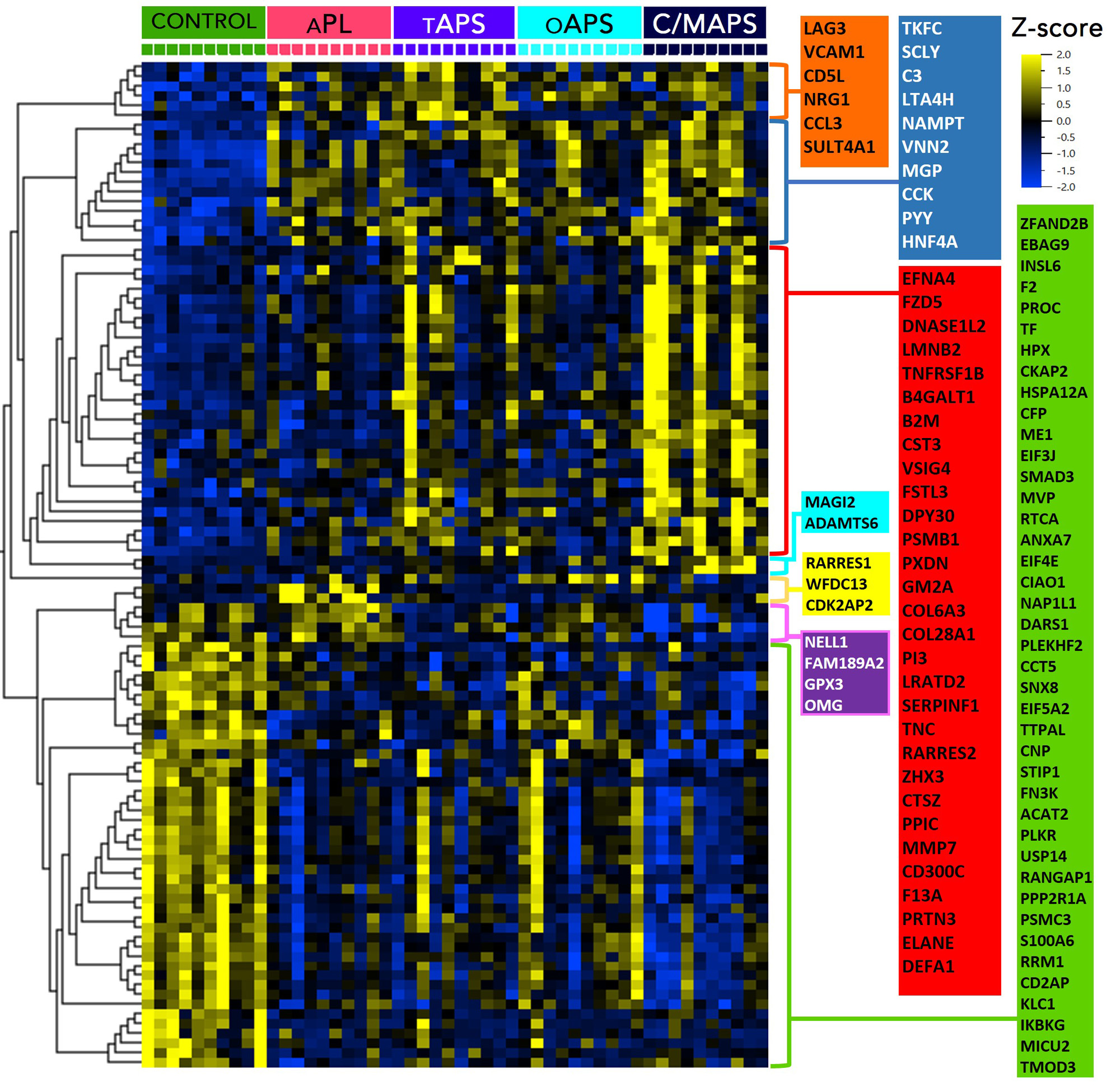
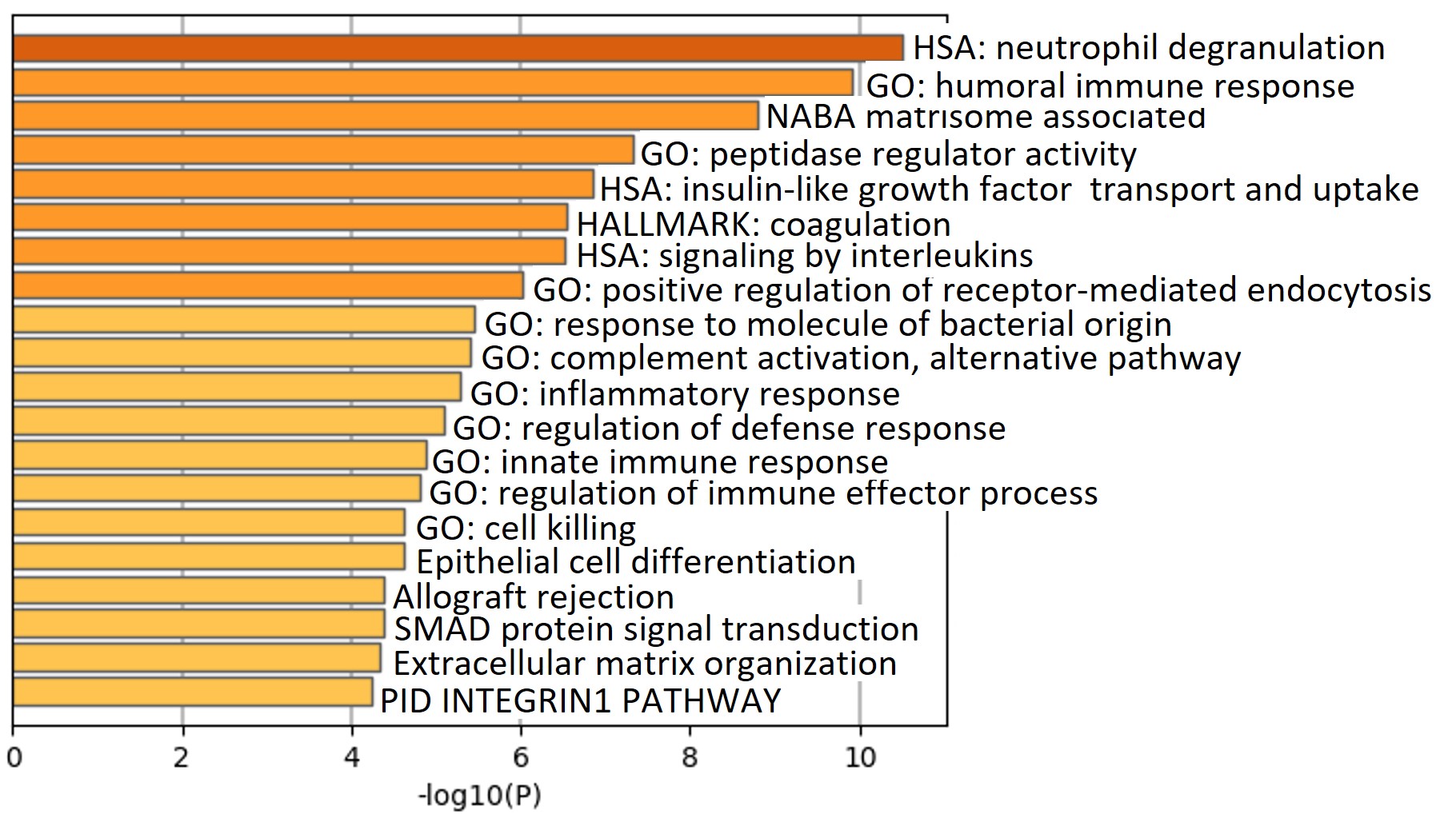
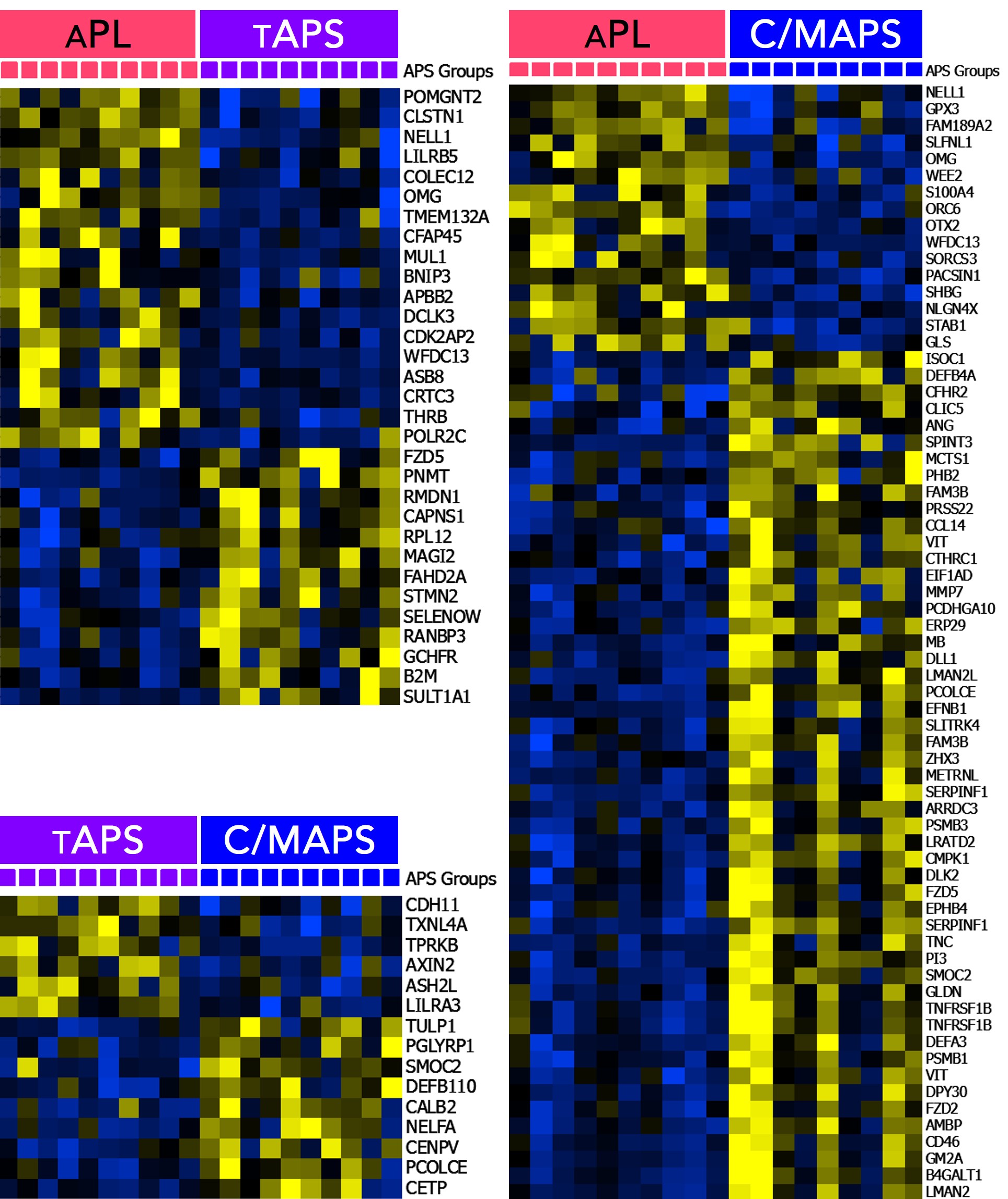
Results: The median age of patients was 48 years; 30% were men, 70% had triple aPL-positivity, and no one had a concurrent diagnosis of lupus. A set of concordant and differentially abundant proteins clustered patients with 4 APS clinical phenotypes and controls (Figure A) with a high statistical significance (p < 0.0007) and a high false discovery confidence (q < 0.05). Pathway enrichment analysis of proteins in the identified set revealed involvement of several pathways such as neutrophil degranulation (p < 10-10), humoral immune response (p < 10-9), coagulation (p < 10-6), alternative complement (p < 10-5) and others (Figure B). Pairwise analyses of APS subtypes revealed increasing abnormalities in these pathways with worsening APS severity, but distinctly in cellular processes such as endocytosis/vesicle-mediated transport, receptor signaling, signal transduction and cellular differentiation (Figure C). This was particularly striking in C/MAPS subtype, with members of negative regulation of inflammatory response and cellular differentiation processes being the only distinction between tAPS and C/MAPS.
Conclusion: Plasma proteome of several APS subtypes is characterized by alteration in peripheral blood cellular processes, particularly receptor signaling, signal transduction and regulation of cellular differentiation, in addition to significant neutrophil, complement, coagulation and cytokine activation notable in all aPL-positive patients.
To cite this abstract in AMA style:
Butt A, Garcia Milian R, Goshua G, Gu S, Chock E, Restrepo V, Hwa J, Chun H, Belmont H, Nina K, Branch D, Petri M, Knight J, Willis R, Bertolaccini M, Erkan D, lee A, Sharda A, Pine A. Plasma Proteomic Profiling in Antiphospholipid Antibody-positive Patients with Different Clinical Phenotypes: Results from the Antiphospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION) Registry [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/plasma-proteomic-profiling-in-antiphospholipid-antibody-positive-patients-with-different-clinical-phenotypes-results-from-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/plasma-proteomic-profiling-in-antiphospholipid-antibody-positive-patients-with-different-clinical-phenotypes-results-from-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international/