Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Plasma calprotectin is a sensitive biomarker of inflammation in patients with rheumatoid arthritis (RA) and reflects activation of granulocytes and macrophages. Calprotectin has not previously been studied in a head-to-head trial of multiple biological mechanisms of action versus active conventional therapy (ACT) with methotrexate and prednisolone over 48 weeks of therapy. The purpose of this study was to assess the effect of treatment on plasma calprotectin levels in patients with early RA by determining the 48-week change in the four arms of the NORD-STAR study, a large multicenter randomized head-to-head clinical trial of ACT versus tumor necrosis factor (TNF) inhibition, T-cell co-stimulation inhibition, and interleukin-6 (IL-6) inhibition, all combined with methotrexate.
Methods: Calprotectin was analyzed in plasma samples at baseline, week 4, week 24 and week 48 from 400 treatment naïve patients from Norway and Sweden with early RA in the NORD-STAR study. Samples were analyzed using a calprotectin ELISA alkaline phosphatase (ALP) kit from CalProLab (Oslo, Norway) in a Dynex DS2 processing system (normal levels < 910 µg/L). Patients were assessed by clinical (CRP, 28 SJC/TJC, physician global) and patient reported assessments. Crude and adjusted linear regression analyses were performed in R 4.3.2 with calprotectin levels at week 24 and 48 as the outcome, and the four treatment arms represented by dummy variables. Calprotectin levels were log10 transformed. The adjustment variables were age, sex, anti-CCP status, country and baseline calprotectin levels.
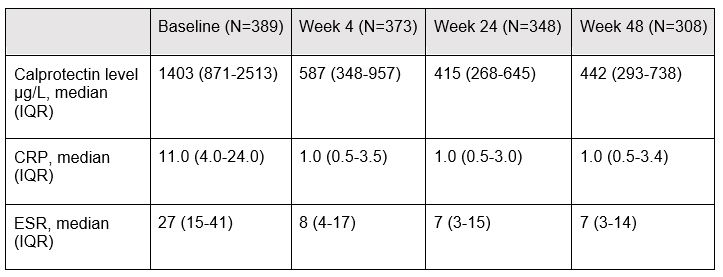
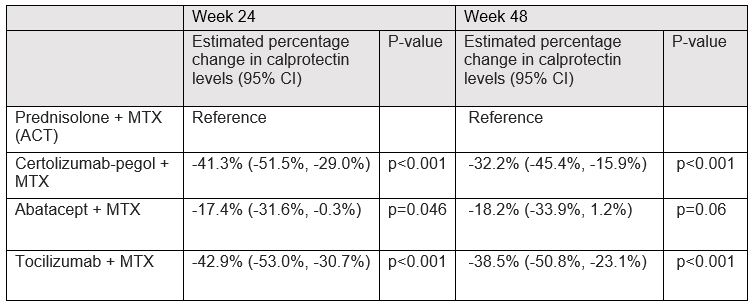
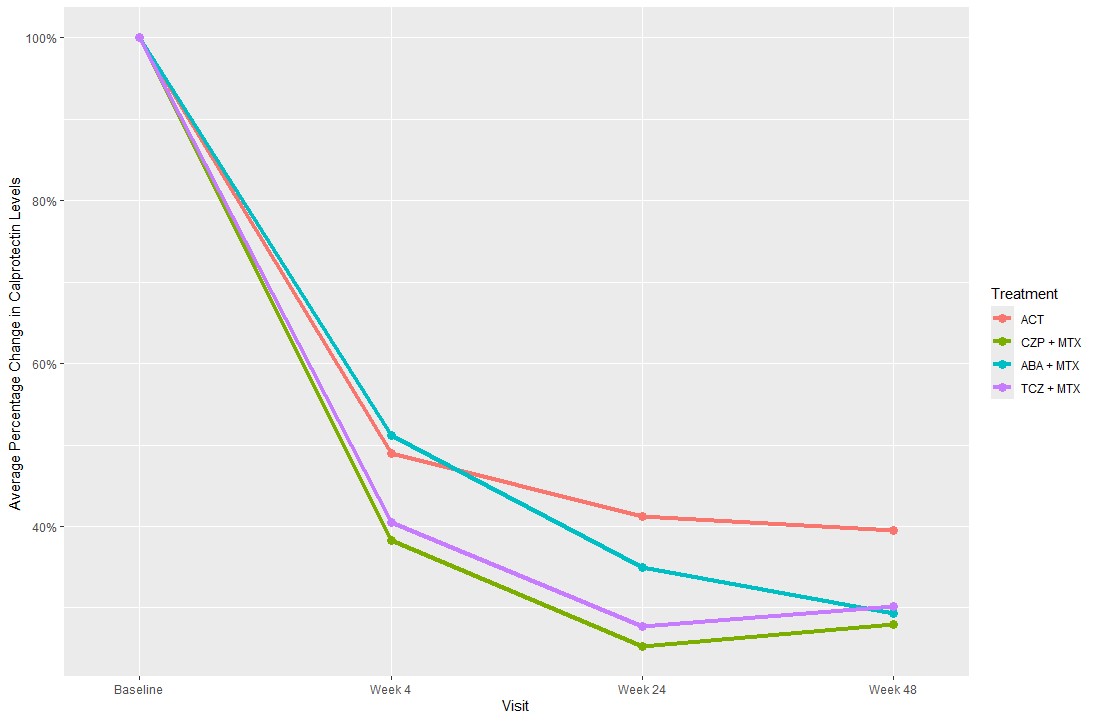
Results: At baseline, the mean time since diagnosis was 15.8 (SD) (23.1) days, mean age 53.7 (15.0) years, 81% were anti-CCP positive and 69.2% female. Median calprotectin levels were 1403µg/L (interquartile range (IQR)) (871-2513) at baseline, 587µg/L (348-957) at week 4, 415µg/L (268-645) at week 24, and 442µg/L (293-738) at week 48 (Table 1). Treatment with CZP+MTX, ABA+MTX and TCZ+MTX were associated with a mean decrease in calprotectin levels at week 24 of 41.3% (95% CI [-51.5%, -29.0%]), 17.4% (95% CI [-31.6%, -0.3%]) and 42.9% (95% CI [-53.0%, -30.7%]), respectively, compared to treatment with ACT (Table 2). For week 48, treatment with CZP+MTX and TCZ+MTX were associated with a statistically significant decrease in calprotectin levels of 32.2% (95% CI [-45.4%, -15,9%]) p< 0.001 and 38.5% (95% CI [-50.8%, -23.1%]) p< 0.001, respectively, compared to treatment with ACT. However, no statistically significant difference in calprotectin levels was observed in patients treated with ABA+MTX -18.2%, (95% CI [-33.9%, 1.2%]) p=0.06, compared to ACT.
Conclusion: Plasma calprotectin levels in early RA declined significantly at week 24 in patients treated with any biological mechanism of action combined with methotrexate versus ACT; however, at week 48 a statistically significant decrease in calprotectin levels was only observed in patients treated with a TNF inhibitor or an IL-6 inhibitor when compared to ACT. These results suggest that plasma calprotectin reflects specific inflammatory pathways rather than overall inflammation.
ACT: active conventional therapy, CZP+MTX: certolizumab-pegol and methotrexate, ABA+MTX: abatacept and methotrexate, TCZ+MTX: tocilizumab and methotrexate
To cite this abstract in AMA style:
Stevens D, Sundbakk L, Kazemi A, Heiberg M, Sokka-Isler T, Gudbjornsson B, Lend K, Rudin A, Nurmohamed M, Lampa J, Hetland M, Ostergaard M, Van Vollenhoven R, Uhlig T, Haavardsholm E, Hammer H. Plasma Calprotectin Assessed in Multiple Biological Treatment Strategies for Early Rheumatoid Arthritis over 48 Weeks [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/plasma-calprotectin-assessed-in-multiple-biological-treatment-strategies-for-early-rheumatoid-arthritis-over-48-weeks/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/plasma-calprotectin-assessed-in-multiple-biological-treatment-strategies-for-early-rheumatoid-arthritis-over-48-weeks/