Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: JNJ-77242113, a targeted oral peptide, inhibits IL-23 signaling by binding the IL-23 receptor. At all doses, JNJ-77242113 showed superior efficacy at Week 16 versus placebo (PBO) in moderate-to-severe psoriasis in FRONTIER-1.1 FRONTIER-2 was a multicenter, long-term extension, double-blind, dose-ranging, phase 2b study evaluating efficacy and safety of JNJ-77242113 in adults with moderate-to-severe plaque psoriasis who were candidates for systemic treatment or phototherapy.
Methods: FRONTIER-1 randomized patients 1:1:1:1:1:1 to JNJ-77242113 25 mg once daily (QD), 25 mg twice daily (BID), 50 mg QD, 100 mg QD, 100 mg BID, or PBO through Week 16. In FRONTIER-2, patients completing FRONTIER-1 (at Week 16) continued their assigned dose through Week 52; those randomized to PBO crossed over to 100 mg QD (PBO→100 mg QD). The primary endpoint was the proportion of patients achieving ≥75% improvement in Psoriasis Area and Severity Index (PASI 75) at Week 52. Response rates were estimated using non-responder imputation and FRONTIER-1 baseline data.
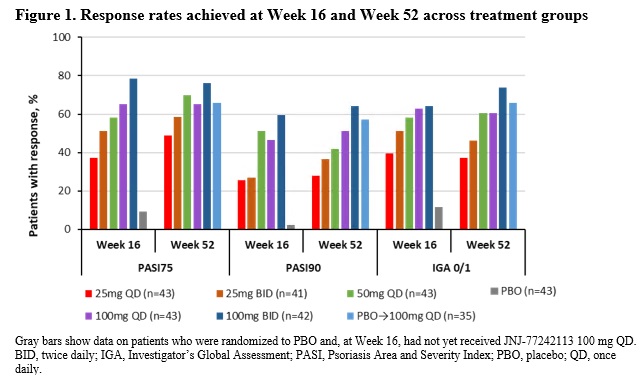
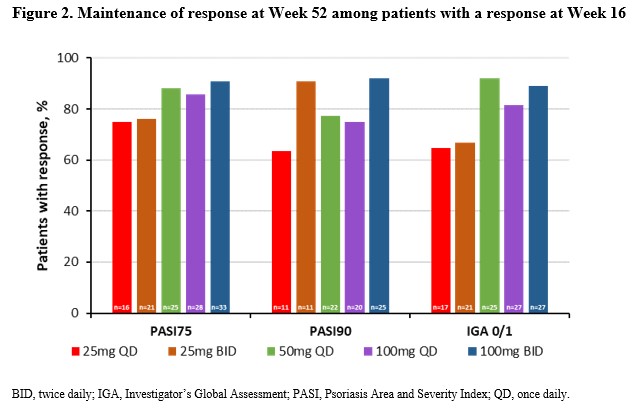
Results: Response rates among JNJ-77242113-treated patients from FRONTIER-1 were maintained, across dose groups, through Week 52 (Figure 1). Patients who crossed over to JNJ-77242113 from PBO at Week 16 (PBO→100 mg QD) had substantially higher response rates at Week 52 (Figure 1). At Week 52, proportions of patients achieving PASI 75 were JNJ-77242113: 25 mg QD 48.8%, 25 mg BID 58.5%, 50 mg QD 69.8%, 100 mg QD 65.1%, 100 mg BID 76.2%, and PBO→100 mg QD 65.7%; respective rates for ≥90% improvement in PASI (PASI 90)/complete clearance (PASI 100) were 27.9%/14.0%, 36.6%/17.1%, 41.9%/20.9%, 51.2%/25.6%, 64.3%/40.5%, and 57.1%/34.3%. Proportions of patients achieving an Investigator’s Global Assessment (IGA) score of 0/1 or 0 were JNJ-77242113 at Week 52 were: 25 mg QD 37.2%/14.0%, 25 mg BID 46.3%/19.5%, 50 mg QD 60.5%/23.3%, 100 mg QD 60.5%/30.2%, 100 mg BID 73.8%/42.9%, and PBO→100 mg QD 65.7%/31.4%. Approximately 90% of patients receiving JNJ-77242113 100 mg BID who had achieved a PASI 75, PASI 90, or IGA 0/1 response at Week 16 maintained the response at Week 52 (Figure 2). Across dose groups, 58.6% of patients experienced adverse events (AEs), with no evidence of a dose-dependent increase in AEs, including gastrointestinal disorders. The proportion of patients with serious AEs through Week 52 was 4% and all serious AEs were considered unrelated to study treatment.
Conclusion: In psoriasis patients receiving JNJ-77242113, the first targeted oral peptide to selectively block IL-23 pathway signaling, rates of near-complete/complete skin clearance from FRONTIER-11 were maintained through Week 52; the highest response rates were seen in patients randomized to JNJ-77242113 100 mg BID. Among patients who had achieved a PASI or IGA response at Week 16, responses were maintained in substantial proportions of patients at Week 52. Consistent with prior studies, no safety signals were identified.
Reference:
1. Bissonnette R, et al. N Engl J Med. 2024;390:510-21.
To cite this abstract in AMA style:
K. Ferris L, Bagel J, Huang Y, Pink A, Tyring S, Kokolakis G, DeLozier A, Kakaley D, Li S, Shen Y, Ota T, Bissonnette R. Phase 2b, Long-term Extension, Dose-ranging Study of Oral JNJ-77242113 for the Treatment of Moderate-to-Severe Plaque Psoriasis: FRONTIER-2 [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/phase-2b-long-term-extension-dose-ranging-study-of-oral-jnj-77242113-for-the-treatment-of-moderate-to-severe-plaque-psoriasis-frontier-2/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/phase-2b-long-term-extension-dose-ranging-study-of-oral-jnj-77242113-for-the-treatment-of-moderate-to-severe-plaque-psoriasis-frontier-2/