Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Janus Kinase (JAK) inhibitors are the agents of choice for the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis when other DMARDs are ineffective. However, they are associated with increased risks of cerebrovascular accident, cardiac failure, venous thromboembolism, malignancy, pericarditis, hyperlipidemia, infection, duodenal ulcer perforation, liver injury, and type 2 diabetes mellitus. Tofacitinib (Xeljanz) was FDA approved in 2012 and Upadacitinib (Rinvoq) in 2019. As with many medications, JAK inhibitors carry a risk of side effects. We sought to investigate difference in side-effect profiles between Tofacitinib and Upadacitinib.
Methods: The FAERS system gathers information on adverse events and medication errors. We assessed real-world data through the FAERS database and post-marketing reports.
A pharmacovigilance study on AEs associated with Tofacitinib and Upadacitinib using the publicly available FAERS was performed. Disproportionality analysis by calculating the reporting odds ratio (ROR) was reported. Post-marketing reports between 2020 and 2024 were analyzed.
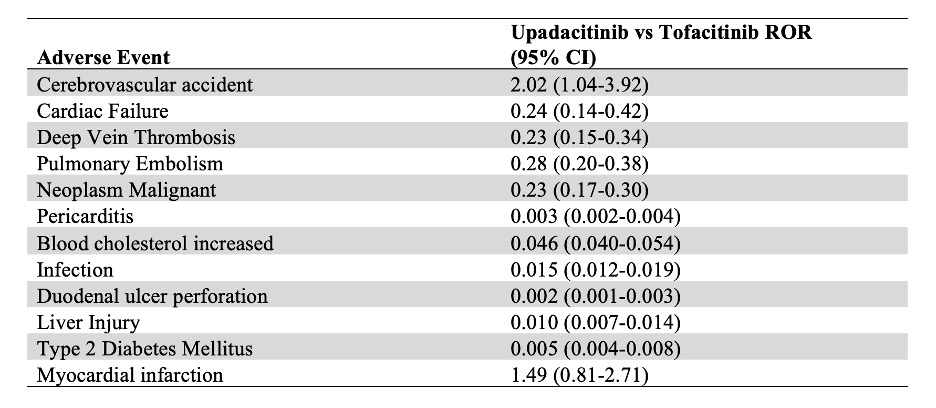
Results: As of June 2024, there were a total of 2,145 and 42,490 reported AEs for Tofacitinib and Upadacitinib, respectively. Upadacitinib had a higher risk of cerebrovascular accident compared to Tofacitinib (ROR 2.02, 95% CI 1.04-3.92). Conversely, Upadacitinib showed a weaker association of deep vein thrombosis (ROR 0.23, 95% CI 0.15-0.34), pulmonary embolism (ROR 0.28, 95% CI 0.20-0.38), malignant neoplasm (ROR 0.23, 95% CI 0.17-0.30), cardiac failure (ROR 0.24, 95% CI 0.14-0.42) pericarditis (ROR 0.003, 95% CI 0.002-0.004), hyperlipidemia (ROR 0.046, 95% CI 0.040-0.054), infection (ROR 0.015, 95% CI 0.012-0.019), duodenal ulcer perforation (ROR 0.002, 95% CI 0.001-0.003), liver injury (ROR 0.010, 95% CI 0.007-0.014), and type 2 diabetes mellitus (ROR 0.005, 95% CI 0.004-0.008) than Upadacitinib. There was no statistically significant difference in risk of myocardial infarctions amongst the two drugs.
Conclusion: This FAERS study highlights the differential risk profiles of Tofacitinib and Upadacitinib regarding adverse events such as cerebrovascular accidents, cardiac failure, venous thromboembolism, and malignancy. Upadacitinib has a higher risk of cerebrovascular accidents compared to Tofacitinib. Upadacitinib shows lower risks of deep vein thrombosis, pulmonary embolism, malignant neoplasms, and cardiac failure, pericarditis, blood cholesterol increase, infection, duodenal ulcer perforation, liver injury, and type 2 diabetes mellitus than Upadacitinib. These findings stress the need to consider patient-specific risks when choosing between these JAK inhibitors. Further research is needed to explore the underlying mechanisms and long-term impacts of these adverse events to optimize therapeutic strategies for patients with autoimmune conditions.
To cite this abstract in AMA style:
Barlas N, Barlas S, Milutinovic S, Basnyat S. Pharmacosurvellience Study of FDA Adverse Event Reporting System (FAERS) Events of Tofacitinib and Upadacitinib [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/pharmacosurvellience-study-of-fda-adverse-event-reporting-system-faers-events-of-tofacitinib-and-upadacitinib/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacosurvellience-study-of-fda-adverse-event-reporting-system-faers-events-of-tofacitinib-and-upadacitinib/