Session Information
Date: Saturday, November 16, 2024
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Many cytokines implicated in lupus pathogenesis depend on JAK signaling to exert their intracellular action. Tofacitinib (TOFA) inhibits signaling by cytokine receptors associated with JAK3 and/or JAK1 with functional selectivity over receptors that signal via pairs of JAK2. TOFA is approved for the treatment of rheumatoid, psoriatic arthritis, polyarticular juvenile idiopathic arthritis, ankylosing spondylitis, and ulcerative colitis but is not approved for SLE. The purpose of this study was to assess the pharmacokinetics (PK), effectiveness as well as safety and tolerability of TOFA in young adults with active mucocutaneous SLE manifestations.
Methods: In this OL trial (NCT03288324), patients (pts) aged 18-45 yrs with CLASI-activity (A) scores >8 received TOFA 5mg twice daily (BID) added to stable background therapy for up to 72 weeks (wks). Plasma PK parameters from noncompartmental analysis were derived from the concentration-time profiles collected at the end of wk1. Safety/tolerability was measured by the number of adverse events (AEs) and serious AEs (SAEs) during the study. Intention to treat analysis (ITT) with the last observation carried forward (LOCF), effectiveness endpoints were least partial response (PR, 20% CLASI-A improvement) and complete response (CR; CLASI-A=0) were used. Other outcomes were BILAG (mucocut, domain only), SLEDAI, CLASI- damage (D) and quality of life (QoL) using the SkinDex-29 (range: 0-100; domains: symptoms, function limitations, emotional distress).
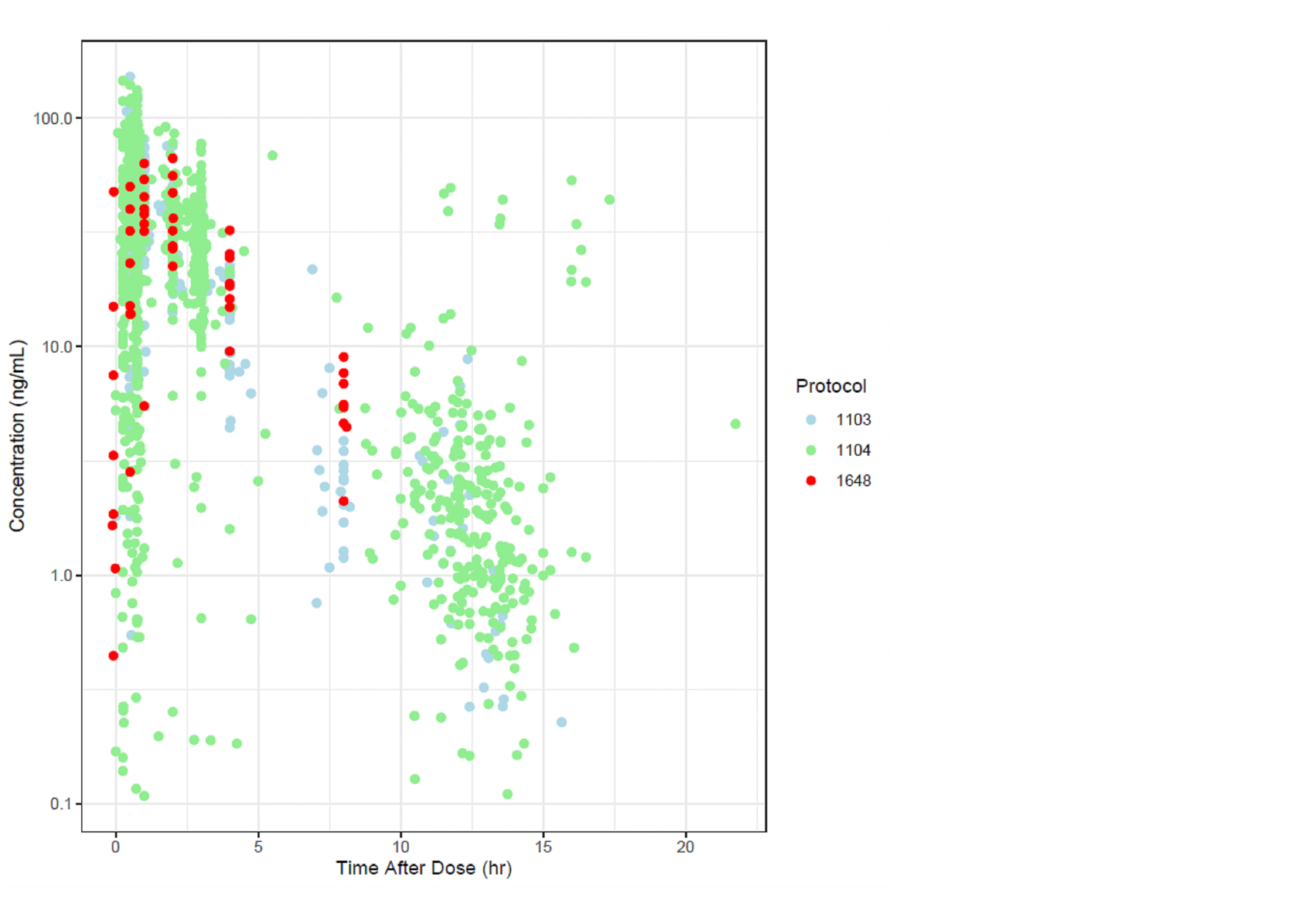
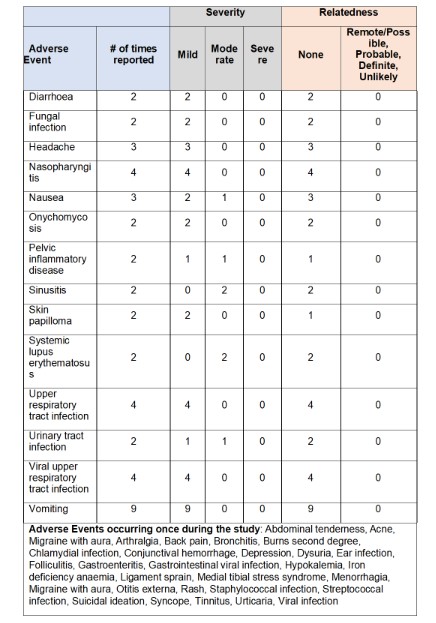
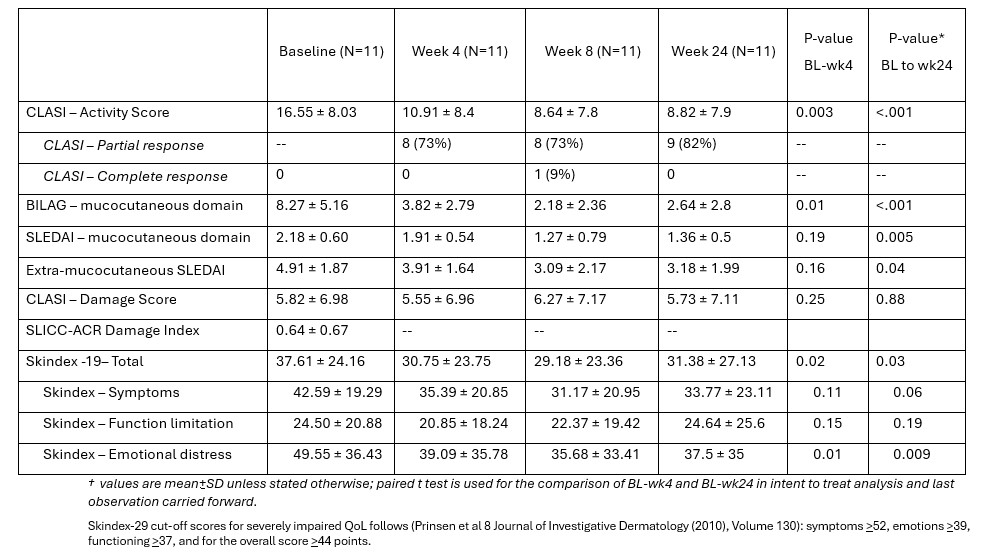
Results: Eleven patients were treated with TOFA. The majority were female (91%, n=10), mean age 23 years, 91% (n=10) were using hydroxychloroquine, 55% (n=6) prednisone and 18% (n=2) used mycophenolate. Three pts discontinued from the study, 1 subject was lost to follow up at week 17 and 2 subjects withdrew from the study at visit 4 and 12. Consent was not withdrawn due to toxicity or adverse events. The PK in SLE was comparable to that observed in juvenile idiopathic arthritis (Fig 1). 72 adverse events were reported (Tbl 1) through the study, with no events were considered related to TOFA. The 16 events had moderate severity (maximum) and all 56 events were considered mild. One AE was serious (pelvic inflammatory disease) but it was unrelated to TOFA. There were no major cardiovascular events, malignancies, or death. As shown in Tbl 3, As shown in Tbl 2, CR was uncommon, but by wk4 73% of the patients had PR. Extracutaneous disease activity also improved significantly. At baseline, there was moderately/severely reduced QoL with severe emotional distress. During the study, all domains of the SkinDex-29 improved with a significant improvement in emotional distress from skin disease (Tbl 2)
Conclusion: Tofacitinib treatment led to rapid improvement of skin findings by week 4. Treatment with TOFA was well tolerated at exposures comparable to those with other rheumatic diseases. Emotional distress from SLE skin manifestations significantly improved on TOFA treatment. Limitations include small sample size.
To cite this abstract in AMA style:
Romankevych I, Singer N, Marathe K, Merritt A, Ogbu E, Chen C, Quinlan-Waters M, Pelletier K, Shi H, Wang X, Huang B, brunner h. Pharmacokinetics (PK), Effectiveness and Safety of Open-label (OL) Tofacitinib for the Treatment of Moderate to Severe Skin Involvement in Young Adults with SLE [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/pharmacokinetics-pk-effectiveness-and-safety-of-open-label-ol-tofacitinib-for-the-treatment-of-moderate-to-severe-skin-involvement-in-young-adults-with-sle/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetics-pk-effectiveness-and-safety-of-open-label-ol-tofacitinib-for-the-treatment-of-moderate-to-severe-skin-involvement-in-young-adults-with-sle/