Session Information
Date: Sunday, November 13, 2016
Title: Metabolic and Crystal Arthropathies - Poster I: Clinical Practice
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Verinurad (RDEA3170) is a novel selective uric acid reabsorption inhibitor in clinical development for the treatment of gout and asymptomatic hyperuricemia. This Phase 1, single-blind, multiple dose, drug-drug interaction (DDI) study evaluated the pharmacokinetics (PK), pharmacodynamics, and tolerability of verinurad in combination with febuxostat (FBX) in healthy male volunteers.
Methods: Subjects were randomized to receive once-daily doses of FBX or verinurad or placebo alone for 7 days, FBX + verinurad or FBX + placebo on days 8−14, and the alternative single agent (FBX or verinurad or placebo) on days 15−21. Subjects received either the combination of verinurad 10 mg + FBX 40 mg or verinurad 2.5 mg + FBX 80 mg. Serial plasma/serum and urine samples were drawn at predetermined time points on Days 7, 14 and 21, and assayed for verinurad, FBX, and uric acid. Baseline samples were drawn on Day −1. Safety was assessed by adverse event (AE) reports, laboratory tests, vital signs, and electrocardiograms (ECGs).
Results: Of 23 randomized subjects, 20 completed the study. FBX 40 mg had no apparent effect on the plasma Cmax and AUC for verinurad 10 mg, whereas FBX 80 mg increased the plasma Cmax and AUC for verinurad 2.5 mg by 25% and 33%, respectively. Verinurad had no effect on FBX PK. Renal clearance of verinurad was unchanged by FBX. The mean maximal reduction in serum uric acid (sUA) was 76% with verinurad 10 mg + FBX 40 mg compared with verinurad 10 mg (56%) or FBX 40 mg (49%) alone (Figure 1A) and was 67% with verinurad 2.5 mg + FBX 80 mg compared with verinurad 2.5 mg (38%) or FBX 80 mg (57%) alone (Figure 1B). Consistent with the mechanism of action (MOA) of verinurad, 24-hr fractional excretion of uric acid (FEUA) increased (2.5 mg: 7.6%; 10 mg: 12.8%) vs baseline (6.5% and 6.0%, respectively). Renal clearance of uric acid (CLUR) increased similarly (2.5 mg: 9.0 mL/min; 10 mg: 12.3 mL/min) vs baseline (8.3 and 7.3 mL/min, respectively). The increases were maintained for 24 hours with verinurad 10 mg + FBX 40 mg (FEUA: 11.8%; CLUR: 13.6 mL/min). Consistent with its MOA, FBX 40 mg and 80 mg decreased the amount of uric acid excreted in urine (246 and 221 mg, respectively, vs baseline: 695 and 818 mg), FEUA (4.8 and 4.2%, respectively, vs baseline: 6.3 and 6.5%), and CLUR (4.9 and 5.0 mL/min, respectively, vs baseline: 7.7 and 8.7 mL/min). No serious AEs, discontinuations due to AEs, or clinically significant laboratory or ECG abnormalities were noted during the study.
Conclusion: No DDI was found with the verinurad 10 mg + FBX 40 mg combination and only a modest one with verinurad 2.5 mg + FBX 80 mg. Both combinations were safe and well tolerated and resulted in greater reduction of sUA than either verinurad or FBX alone. These results support the continued development of this novel approach for the treatment of gout and hyperuricemia. 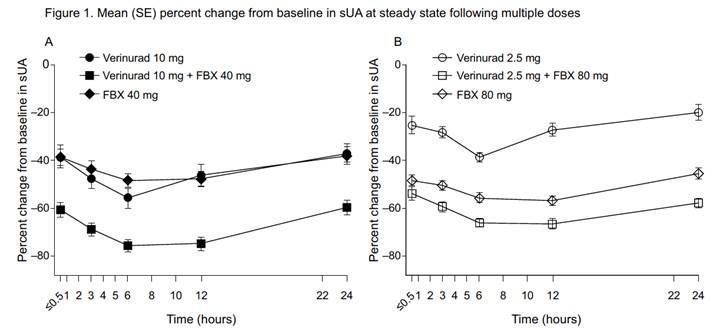
To cite this abstract in AMA style:
VanderLugt J, Gillen M, Yang X, Hall J. Pharmacokinetics, Pharmacodynamics, and Tolerability of Concomitant Multiple Dose Administration of Verinurad (RDEA3170) and Febuxostat in Healthy Adult Male Subjects [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/pharmacokinetics-pharmacodynamics-and-tolerability-of-concomitant-multiple-dose-administration-of-verinurad-rdea3170-and-febuxostat-in-healthy-adult-male-subjects/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetics-pharmacodynamics-and-tolerability-of-concomitant-multiple-dose-administration-of-verinurad-rdea3170-and-febuxostat-in-healthy-adult-male-subjects/
