Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Ustekinumab (UST), an interleukin‑12/23p40 antagonist, is currently approved for juvenile psoriatic arthritis (jPsA) solely in the United States (US). Although a phase 3 study of UST in jPsA (NCT05083182) is underway, patients (pts) with jPsA are rare and difficult to recruit. The UST Pediatric Opportunistic Pharmacokinetic (PK) Study (U-POPS; NCT05252533) was conducted to characterize the PK and safety of UST among pts with jPsA already receiving UST in routine clinical care.
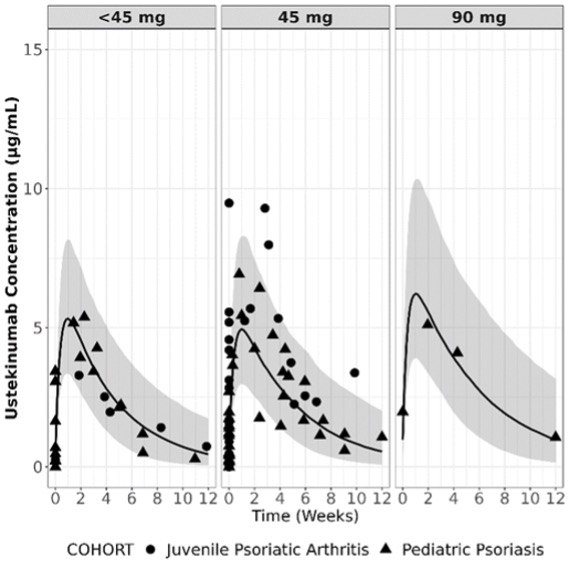
Methods: This real-world open-label study enrolled pts ≥5 to < 18 years old with jPsA (ILAR or Vancouver criteria) and/or pts ≥6 to < 18 years old with pediatric psoriasis (PsO) who had initiated UST ≥16 weeks and had received ≥3 doses prior to enrollment (i.e., should have attained steady-state UST concentrations). The PsO cohort was an internal control group for comparison with previously obtained PsO PK data. UST was administered at the dose and frequency prescribed by their physician. PK samples were drawn ≥7 days apart over a maximum of 4 visits for up to 20 weeks. The primary endpoint was observed UST concentrations. Observed serum UST concentrations were plotted with model-predicted concentrations using a previously developed population PK model developed using adolescent and pediatric PsO pts. Safety data were also collected and are summarized descriptively.
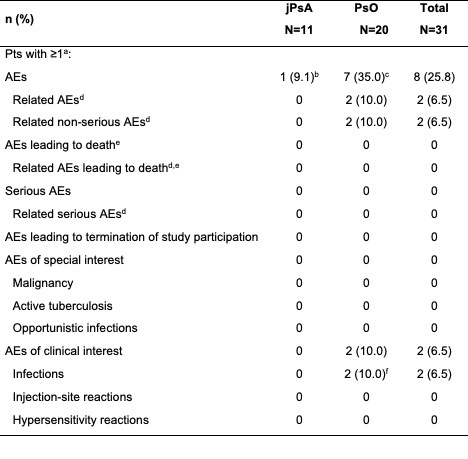
Results: A total of 11 pts with jPsA (mean [SD] age, 15.1 [1.5] years; female, 72.7% [n=8]); White, 72.7% [n=8]) and 20 pts with PsO (mean [SD] age, 12.6 [3.3] years; female, 75.0% [n=15]); White, 60.0% [n=12]) provided 100 UST PK samples after enrollment at 8 US sites from 2022-2023. The median [range] UST dose was 45 mg [18-90 mg] administered every 12 weeks for both pt cohorts. Observed UST serum concentration-time course profiles in pts with jPsA and PsO were adequately captured when overlaid against model‑predicted concentration-time profiles produced using a previously developed population PK model developed for the adolescent and pediatric population with PsO with or without jPsA (Figure). Overall, adverse events (AEs) were reported for 25.8% (jPsA, n=1; PsO, n=7) of pts (Table). AEs of clinical interest included 2 infections (ear infection, influenza) in the PsO cohort; none were reported in the jPsA cohort. No serious AEs, serious infections, malignancies, injection site and/or hypersensitivity reactions, deaths, or AEs leading to termination of study participation were reported. Safety data were consistent with that observed in other pediatric and adult UST trials.1,2
Conclusion: This opportunistic sampling of real-world pts with jPsA who were already receiving UST in routine clinical care led to efficient and reliable data collection from this difficult-to-recruit population. Observed PK exposure and time course from pts with jPsA and pediatric PsO treated with UST was adequately captured using a previously developed population PK model in adolescent and pediatric PsO pts. The model was further validated with pediatric PsO pts on UST from the current study, suggesting that UST exposure and time course are similar between both populations.
1. Landells I, et al. J Am Acad Dermatol. 2015;73(4):594-603.
2. Papp KA, et al. The Lancet. 2008;371(9625):1675-1684.
Abbreviations: AE=adverse event; HLA-B*27=human leukocyte antigen B27; jPsA=juvenile psoriatic arthritis; PsO=pediatric psoriasis; pt(s)=patient(s).
Note: All AEs occurring after signing of informed consent form were considered treatment emergent.
(a) Pts were counted only once for any given AE category, regardless of the number of times they actually experienced the AE in that category.
(b) One pt had an AE of arthritis and an AE of an HLA-B*27 positive lab result.
(c) AEs included the following: vomiting (n=3), abdominal pain (n=2), diarrhea (n=2), nausea (n=2), cough (n=1), nasal congestion (n=1), wheezing (n=1), ear infection (n=1), influenza (n=1), nasopharyngitis (n=1), headache (n=1), syncope (n=1), dysmenorrhea (n=1), and psoriasis (n=1).
(d) An AE is assessed by the investigator as related to study agent.
(e) AEs leading to death are based on AE outcome of ‘fatal.’
(f) AEs included the following: ear infection (n=1) and influenza (n=1).
To cite this abstract in AMA style:
Bishop C, Lam E, Liva S, Leu J, Paller A, Diaz L, Wine Lee L, Rubin C, Carrasco R, Imundo L, Majlessi A, Berezny K, Lomax K, Smith V, Zhang R, brunner h. Pharmacokinetics of Ustekinumab in Patients with Juvenile Psoriatic Arthritis in a Real‑World Opportunistic Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/pharmacokinetics-of-ustekinumab-in-patients-with-juvenile-psoriatic-arthritis-in-a-real%e2%80%91world-opportunistic-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetics-of-ustekinumab-in-patients-with-juvenile-psoriatic-arthritis-in-a-real%e2%80%91world-opportunistic-study/