Session Information
Date: Tuesday, October 28, 2025
Title: (1990–2014) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Pegloticase is approved for uncontrolled gout as 8-mg infusions admixed in 250 cc of normal saline over 120 minutes or more administered every 2 wks with methotrexate (MTX) coadministration. Given the logistical burden of 2-hr infusions every 2 wks and normal saline supply disruptions, the AGILE study evaluated faster 60-minute infusions every two wks with pre-mixed 8 mg pegloticase, in 50 cc ready-to-use (RTU) vials, with MTX co-administration. The overall results of the AGILE study demonstrated a comparable efficacy and safety profile to that of the standard infusion formulation,1 but the pharmacokinetic (PK) results have not been reported.
Methods: The AGILE study was a phase 4, multicenter, open-label study including patients with uncontrolled gout (oral ULT failure or intolerance, SU ≥6 mg/dL, more than one tophus, more than one flare/yr, or gouty arthritis). Key exclusion criteria were contraindication to MTX, G6PD deficiency, serious bacterial infection, eGFR < 40 mL/min/1.73m², or elevated liver function tests. Eligible patients underwent a 4-wk oral MTX run-in of 15 mg weekly with 1 mg daily of folic acid then 24 wks of pegloticase with MTX co-administration. Three cohorts were enrolled receiving 60-, 45-, and 30-minute infusions. Ultimately the 60-minute group was deemed optimal and fully enrolled based on safety reviews. The PK analysis set included all patients who received ≥1 dose of pegloticase and had a post-pegloticase sample evaluable for PK analysis. PK parameters (Cmin, Cmax) were analyzed at week 12, including pre- and post-infusion concentrations.
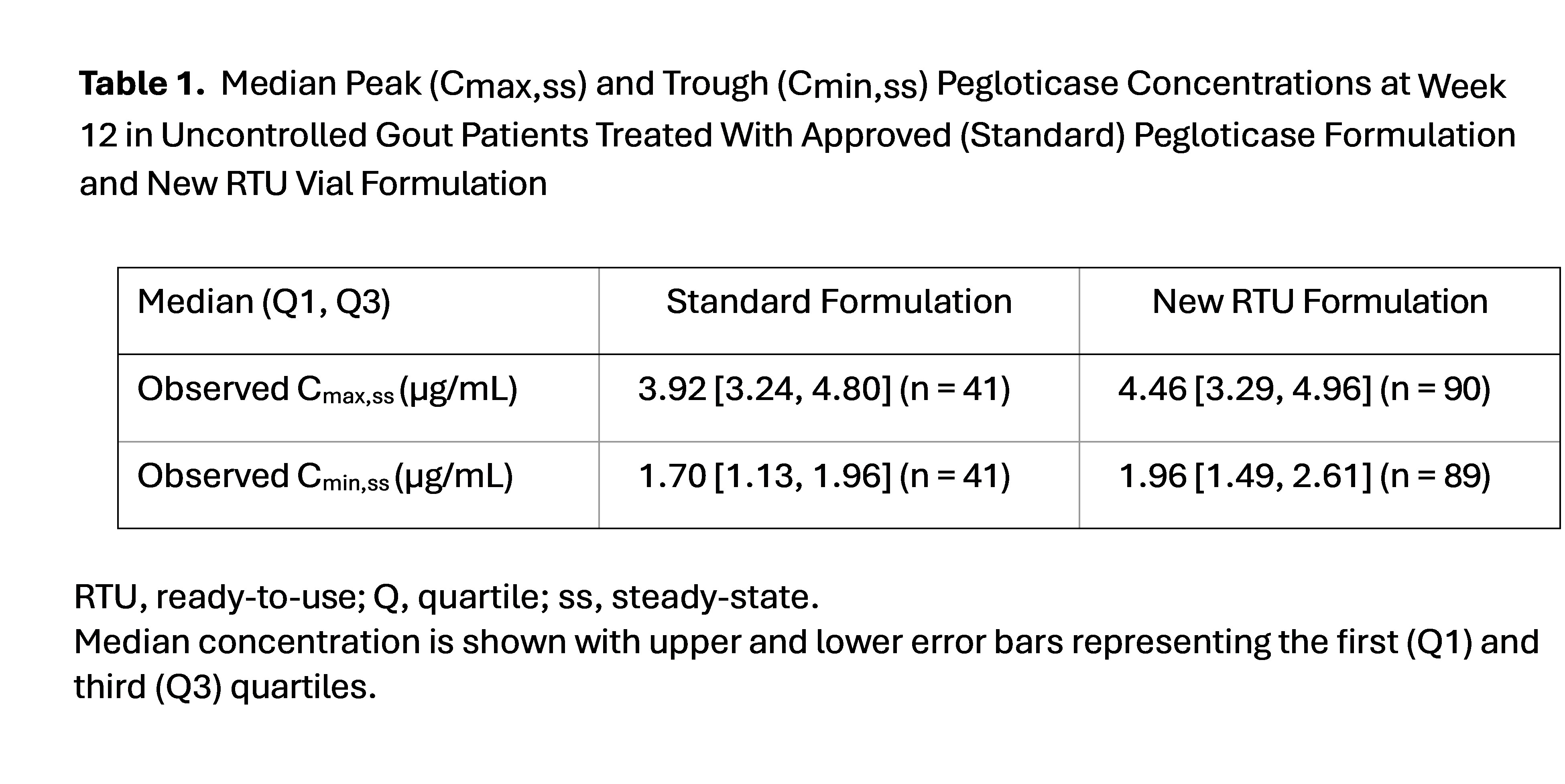
Results: Efficacy and safety of AGILE have been reported among the 60-minute cohort (n = 116), showing an infusion reaction rate of 6% (95% CI, 2.5%-12.0%, 7/116) and SU efficacy response rate at month 6 of 67.2% (95% CI, 57.9%-75.7%, 78/116), which were comparable to standard pegloticase with MTX treatment.1,2 PK analyses included all 3 infusion cohorts and occurred at study week 12, showing a median Cmax of 4.46 (Q1 of 3.29, Q3 of 4.96, n = 90) for RTU vs 3.92 (Q1 3.24, Q3 4.80, n = 41) for standard pegloticase. The Cmin followed the same trend, with median Cmin of 1.96 (Q1 of 1.49, Q3 of 2.61, n = 89) vs 1.70 (Q1 of 1.13, Q3 of 1.96, n = 41) for standard pegloticase. (Table 1, Fig. 1). These differences were small relative to overall exposure variability and not considered clinically significant. Overall, the RTU formulation showed an 11.7% higher geometric mean simulated exposure compared to standard pegloticase (clinically insignificant). Of note, 5 patients (60-minute cohort [n=1]; 30-minute cohort [n=4]) received standard admixed pegloticase and RTU formulation during the study; the PK parameters and response rates in these patients were comparable to the pegloticase RTU and standard formulations.
Conclusion: Pegloticase given in RTU form of premixed 50 cc with 8 mg of pegloticase coadministered with MTX over 60 minutes showed comparable safety, efficacy, and PK results to standard pegloticase. Among a small number of patients that switched from standard pegloticase to RTU formulation, no change in PK was seen.References 1. Troum et al. Arthritis Rheumatol. 2024; 76 (suppl 9). 2. Botson et al. Arthritis Rheumatol. 2023;75:293-304.
To cite this abstract in AMA style:
Troum O, Botson J, Mohammad A, Yang X, Roe N, Verma S, Lamoreaux B. Pharmacokinetics of Ready-to-Use Pegloticase Formulation Compared to Standard Pegloticase Dosing: Data from the AGILE Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/pharmacokinetics-of-ready-to-use-pegloticase-formulation-compared-to-standard-pegloticase-dosing-data-from-the-agile-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetics-of-ready-to-use-pegloticase-formulation-compared-to-standard-pegloticase-dosing-data-from-the-agile-study/

.jpg)