Session Information
Date: Monday, November 13, 2023
Title: (1100–1123) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: In early phase studies AR882 exhibited good dose proportionality, long half-life and dose-dependent serum urate (sUA) lowering effect in a broad range of doses in healthy subjects and gout patients. In a phase 2b study, following once-daily dosing up to 3 months, the pharmacokinetics (PK) and pharmacodynamics (PD), including sUA lowering effect of AR882, were evaluated in all patients; in a subset of these patients more frequent laboratory monitoring was done.
Methods: In the phase 2b, 12-week study, 140 subjects were enrolled into three treatment groups: placebo (n=47), AR882 50 mg (n=46) and AR882 75 mg (n=47) at approximately 1:1:1 ratio. A single blood sample was collected for PK/PD assessment at each clinical visit (every 2 weeks) through Week 12. In addition, a total of 25 gout patients participated in an optional sub-study with more frequent sampling occurring on baseline, Week 2, and Week 8 visits.Serial blood samples were collected up to 24 hours post-dose for AR882 and sUA measurements.
Results: Following long-term treatment, AR882 showed stable exposure across the entire treatment period. At steady-state, AR882 was readily absorbed with median Tmax of 1.50 to 3.50 hours post-dose. AR882 demonstrated dose-dependent increase in plasma exposure. Average half-life of AR882 ranged between approximately 14 and 22 hours. Its active metabolite AR896 was formed with median Tmax between 5- and 8-hours post-dose. Consistent with observations in the early phase studies, AR896 constituted of approximately 9% of Cmax and 10-12% of AUC exposures with longer half-life (29-36 hours) than the parent drug.
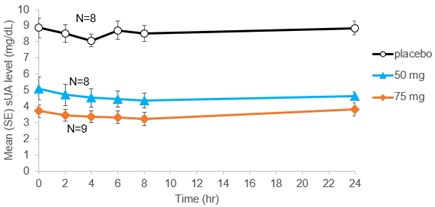
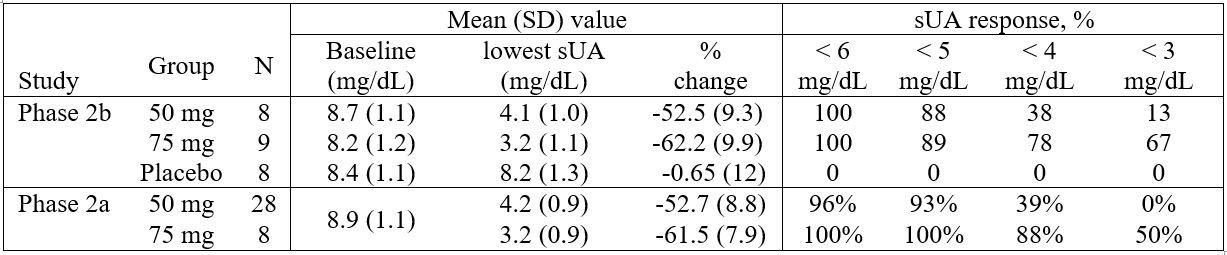
In the sub-study group with more frequent sampling, sUA lowering effect in both AR882 treatment groups showed a smooth, consistent and flat profile across 24 hours with the lowest mean sUA reduced from baseline 8.6 mg/dL to 4.1 mg/dL (-53%) in the 50 mg group and to 3.2 mg/dL (-62%) in the 75 mg group (Figure 1). sUA lowering effects and the response rates in these patients were similar to those observed in an earlier, well-controlled, 3-week, cross-over design phase 2a study (Table 1). Mild or moderate adverse events including gout flares, diarrhea, headache, and upper respiratory infection were observed. None of the AEs led to discontinuation of investigational product.
Conclusion: In a subset of patients with full PK/PD collection, AR882 showed potent sUA lowering effect with similar exposures to those observed in closely monitored early-phase studies. AR882 50 mg and 75 mg doses were well tolerated during the entire study with an unremarkable safety profile.
To cite this abstract in AMA style:
Fleischmann R, Wei J, Shen Z, Morris s, Polvent E, Clouser-Roche A, Hingorani V, Yan R, Yan S, Keenan R, Yeh L. Pharmacokinetics and Pharmacodynamics of AR882 Following 12-Week Treatment in Patients with Gout [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/pharmacokinetics-and-pharmacodynamics-of-ar882-following-12-week-treatment-in-patients-with-gout/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetics-and-pharmacodynamics-of-ar882-following-12-week-treatment-in-patients-with-gout/