Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: This was an exploratory analysis of genetic polymorphisms, plasma biomarkers, and blood leukocytes with clinical response in the phase 3 clinical study CC-10004-BCT-002 (NCT02307513).
Methods: Subjects with active Behçet’s disease (BD) were randomized (1:1) to apremilast (APR) 30 mg BID or placebo (PBO). The primary clinical efficacy endpoint was the oral ulcer area under the curve from Week 0 to 12 (AUCWk0-12). Among 207 subjects enrolled, 140 provided consent for DNA genotyping, 116 for plasma biomarker testing, and 96 for leukocyte subset testing. Genotyping was performed on the Illumina Omni2.5 BeadChip (Covance Genomics Laboratory). TNF-α, IL-6, IFN-γ, and IL-17A levels were measured using Simoa Single Molecule Array; IL-8 and IL-23 were measured using the Human DiscoveryMAP multiplex panel (Myriad RBM). Th17, Treg, and CD3 T cells were counted using bisulfite-specific RT-PCR (Epiontis Gmbh). A rank ANCOVA model focused on between-treatment differences (APR vs. PBO) in % change from baseline for each biomarker/leukocyte subtype over the 12 weeks of treatment. Within each treatment group, the correlation of % change from baseline at Week 12 in biomarker/leukocyte subtype with the primary efficacy endpoint AUCWk0-12 was examined using a univariate regression model. A separate regression model was used to assess the interaction between treatment and the biomarker/leukocyte subtype clinical response.
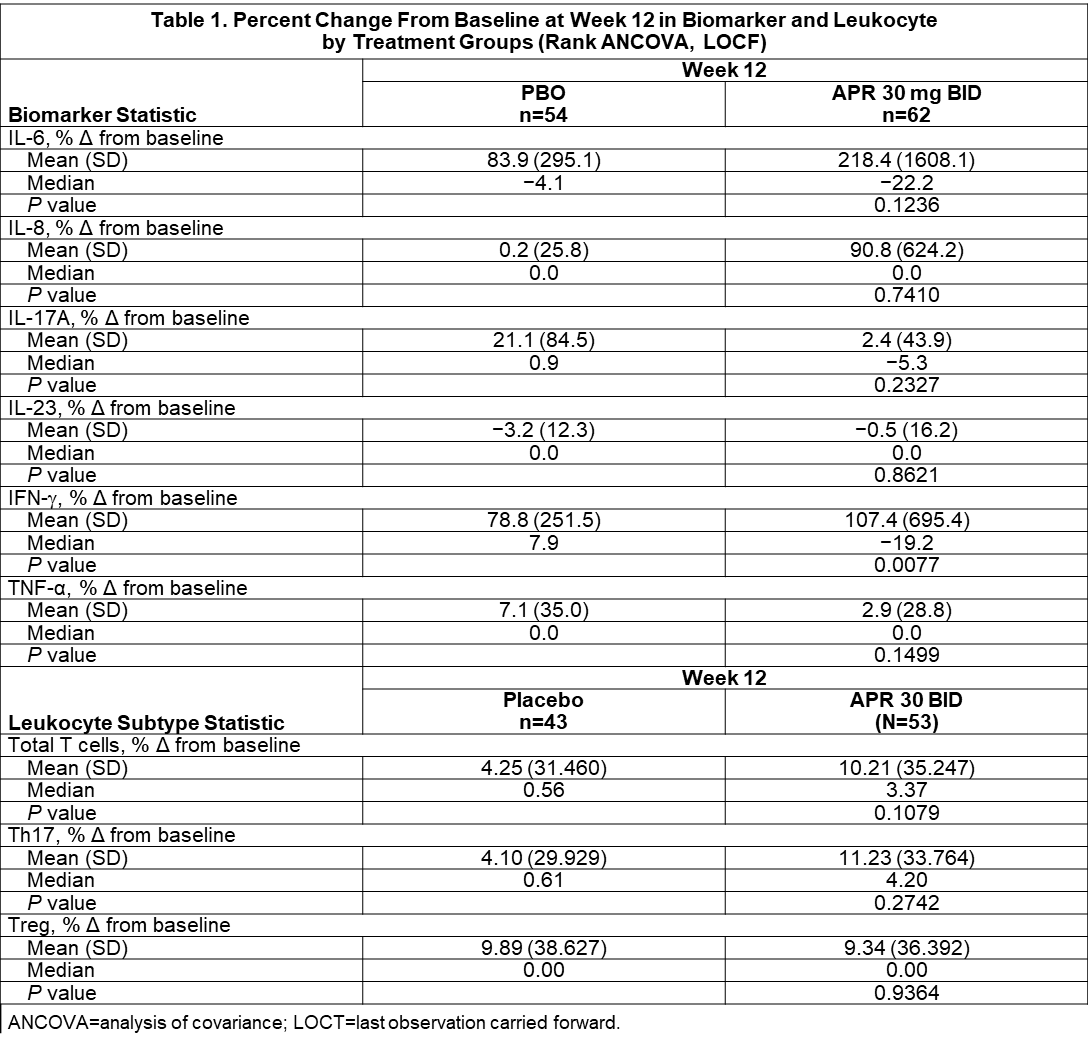
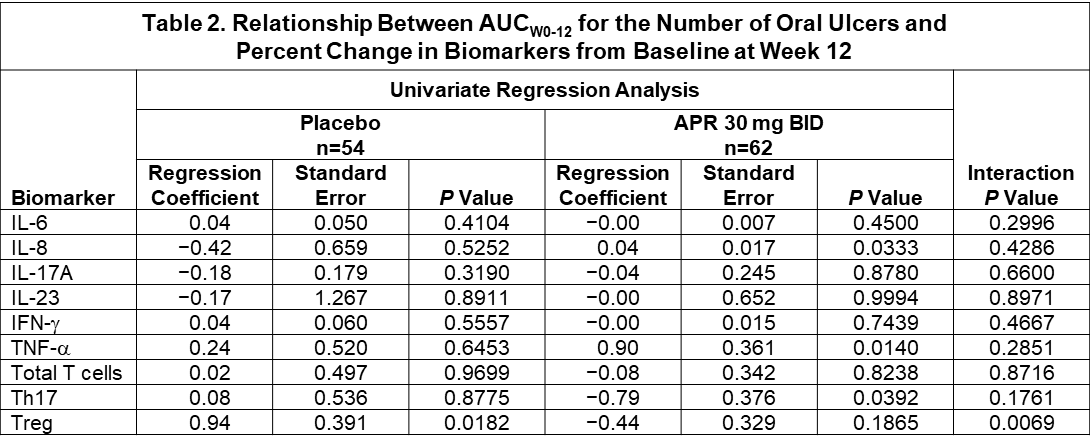
Results: Pharmacogenetic analysis of BD risk variants in HLA-B, IL-10, TLR2, ACE, TNF, GIMAP, PDGFRL, and UBAC2 + 55 genes associated with PDE4 biology yielded no candidate variants that were significantly associated with response to APR or PBO at a Bonferroni-corrected P value of 2 x 10−6. Clinical response to APR with respect to HLA-B51 yielded an OR of 1.21 (95% CI, 0.53-2.75), indicating no significant relationship (Figure 1). Pharmacodynamic changes for IL-6, IL-3, IL-17A, IL-23, and TNF-α were not statistically significant. APR treatment was associated with a significant change in IFN-γ (mean: +107.4%; median: −19.2%) vs. PBO (mean: +78.8%; median: +7.9%) (P=0.0077) (Table 1). Using a univariate regression model, TNF-α showed strong positive correlation with AUCWk0-12 in the APR group (r=0.90; P=0.0140); IL-8 had weak positive correlation with AUCWk0-12 in the APR group (r=0.04; P=0.0333). A significant negative correlation was observed between the % change from baseline in number of Th17 cells and AUCWk0-12 in the APR group (r=−0.79; P=0.0392) and a significant positive correlation was observed with the % change from baseline in number of Treg cells and efficacy in the PBO group (r=0.94; P=0.0182). Of all the biomarkers and leukocyte subtypes examined in a regression model using treatment as a factor, only Treg had a statistically significant treatment interaction (P=0.0069) (Table 2).
Conclusion: Although there were no genetic predictors of clinical response to APR treatment, strong correlation was observed between the % change from baseline in plasma TNF-α with AUCWk0-12 in the APR group. A negative correlation was observed between % change from baseline in Th17 cells and AUCWk0-12 in the APR group and a positive association was observed between Treg cells and AUCW0-12 in the PBO group.
To cite this abstract in AMA style:
Maranville J, Medvedeva I, Yang R, Chen M, Fang L, Collazo S, McCue S, Hochfeld M, Schafer P. Pharmacogenetics and Pharmacodynamics of Response to Apremilast in a Phase 3 Clinical Study in Subjects with Active Behçet’s Disease [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/pharmacogenetics-and-pharmacodynamics-of-response-to-apremilast-in-a-phase-3-clinical-study-in-subjects-with-active-behcets-disease/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacogenetics-and-pharmacodynamics-of-response-to-apremilast-in-a-phase-3-clinical-study-in-subjects-with-active-behcets-disease/