Session Information
Date: Sunday, November 13, 2016
Title: Metabolic and Crystal Arthropathies - Poster I: Clinical Practice
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Verinurad (RDEA3170) is a high-affinity, selective URAT1 inhibitor in development for treatment of gout and asymptomatic hyperuricemia. This Phase 2a, randomized, open-label, multicenter study investigated the multiple-dose pharmacodynamics (PD), pharmacokinetics (PK), and safety of oral verinurad in combination with allopurinol versus allopurinol alone in adults with gout (NCT02498652).
Methods: Patients aged 18-75 years with gout and serum uric acid (sUA) ≥8 mg/dL were randomized to 1 of 2 cohorts to receive allopurinol (300 mg) combined with verinurad (dose range 2.5 mg to 20 mg) and allopurinol 300 mg or 600 mg alone (each treatment period 7 days). Medications were administered once daily ~30 min after breakfast (for allopurinol 300 mg b.i.d. group, the second allopurinol dose was in the evening). Colchicine 0.6 mg for gout flare prophylaxis was initiated at approximately Day -14 (start of urate-lowering therapy [ULT] washout) or Day -7 if not on ULT. Serial blood and urine samples were measured on Days -1, 1, 7, 14, 21, 28, and 35 for PD and PK endpoints. Safety assessments included adverse events (AEs) and laboratory, ECG, and vital sign parameters.
Results: Forty-one patients were randomized (n=20–21 per cohort). Serum PD data pooled across cohorts demonstrated maximal % decrease in sUA from baseline (Emax) at 6-10 h after verinurad and allopurinol combination treatment. Addition of verinurad (2.5 mg to 20 mg) to allopurinol decreased sUA in dose-dependent manner (Figure). Greater sUA reductions were observed for dose combinations of verinurad ≥5 mg with allopurinol 300 mg versus allopurinol 600 mg alone, while allopurinol 600 mg once daily was equivalent to allopurinol 300 b.i.d. Emax was 46.9%, 58.9%, 59.9%, 67.1%, 68.4%, and 74.3% for verinurad at doses of 2.5, 5, 7.5, 10, 15, and 20 mg combined with allopurinol 300 mg, versus 39.7%, 53.8%, and 54.4% with allopurinol 300 mg, allopurinol 600 mg, and allopurinol 300 mg b.i.d. alone. No drug-drug interaction on verinurad and allopurinol plasma PK parameters was observed. Verinurad decreased oxypurinol Cmax by 19.0-32.4% and AUC0-24 by 20.9-39.2%; reductions in oxypurinol Cmax and AUC0-24 were dose dependent, with no further decreases above 15 mg verinurad dose. Verinurad at doses from 2.5 mg to 20 mg was well tolerated, with no serious AEs, withdrawals due to AEs, or renal-related events during combination treatment. Laboratory values and vital signs indicated no clinically meaningful changes. There were no cases of serum creatinine elevation ≥1.5x baseline.
Conclusion: Verinurad coadministered with allopurinol dose-dependently decreased sUA. All dose combinations of verinurad and allopurinol were generally well tolerated with no serious AEs or renal-related events during combination treatment. 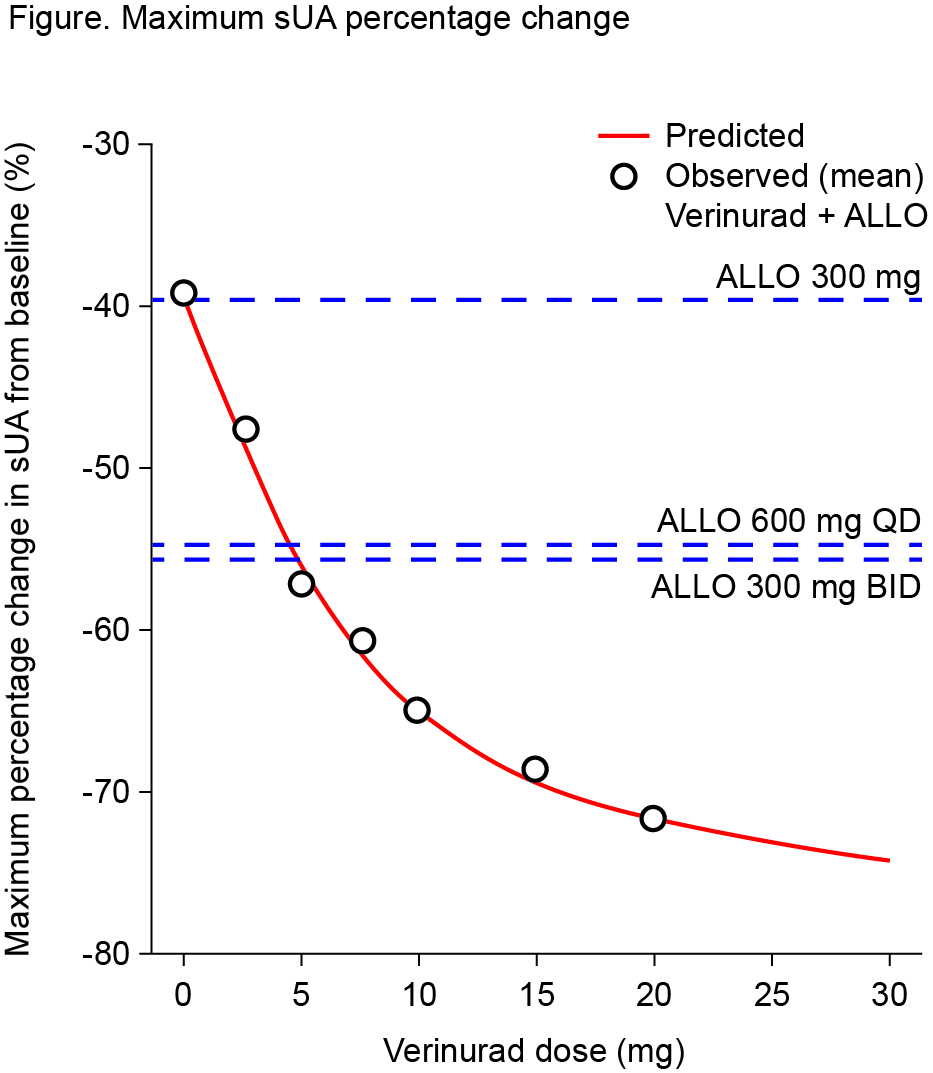
To cite this abstract in AMA style:
Fleischmann R, Winkle P, Hall J, Yan X, Miner JN, Hicks L, Valdez S, Hernandez-Illas M. Pharmacodynamic Effects and Safety of Verinurad in Combination with Allopurinol Versus Allopurinol Alone in Adults with Gout: A Phase 2a, Open-Label Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/pharmacodynamic-effects-and-safety-of-verinurad-in-combination-with-allopurinol-versus-allopurinol-alone-in-adults-with-gout-a-phase-2a-open-label-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacodynamic-effects-and-safety-of-verinurad-in-combination-with-allopurinol-versus-allopurinol-alone-in-adults-with-gout-a-phase-2a-open-label-study/
