Session Information
Date: Sunday, November 13, 2016
Title: Metabolic and Crystal Arthropathies - Poster I: Clinical Practice
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Verinurad (RDEA3170) is a high-affinity, selective URAT1 inhibitor in development for the treatment of gout and asymptomatic hyperuricemia. This Phase 1, single-dose, open-label study investigated the pharmacodynamics (PD), pharmacokinetics (PK), and safety of oral verinurad in adult subjects with mild, moderate, or severe renal impairment and matched controls with normal renal function (NCT02219516).
Methods: Adult males aged 18-85 years were enrolled with a screening serum uric acid (sUA) 4.5-10 mg/dL and creatinine clearance calculated by Cockcroft-Gault formula of 60 to <90 mL/min (mild renal impairment), 30 to <60 mL/min (moderate impairment), 15 to <30 mL/min (severe impairment), or ≥90 mL/min (matched controls). Oral verinurad 15 mg was administered once under fasted conditions. Serial blood and urine samples were taken 30 min before and up to 72 hours postdose. Safety assessments included laboratory, ECG, and vital sign parameters as well as adverse event (AEs) reporting.
Results: PD data were based on 7-8 subjects per group. Verinurad decreased sUA in all groups, with greatest changes in the normal function and mild renal impairment groups (Figure). Mean (SD) maximal % change in sUA from baseline (Emax) was -38.3(14.8)%, -36.9(13.6)%, -20.5(6.64)%, and -12.6(6.94)%, respectively, in the normal function and mild, moderate, and severe renal impairment groups. Increase in the amount of excretion of uric acid due to verinurad treatment decreased in subjects with moderate and severe renal impairment. Plasma Cmax and AUC of verinurad increased with decreasing renal function. Verinurad at the 15 mg dose was well tolerated, with no serious AEs, no subject withdrawals due to AEs, and no Renal Events Adjudication Committee (REAC)-adjudicated renal events during treatment. One patient in each renal impairment group had treatment-emergent AEs considered possibly related to verinurad, which were categorized as gastrointestinal in nature. There were no clinically meaningful changes noted in laboratory values or vital signs.
Conclusion: The sUA lowering effect of verinurad was observed across the spectrum of renal function. Consistent with the verinurad renal-dependent mechanism of action, decreasing sUA lowering was demonstrated with increasing renal impairment. Verinurad safety and tolerability were similar across all stages of renal impairment. 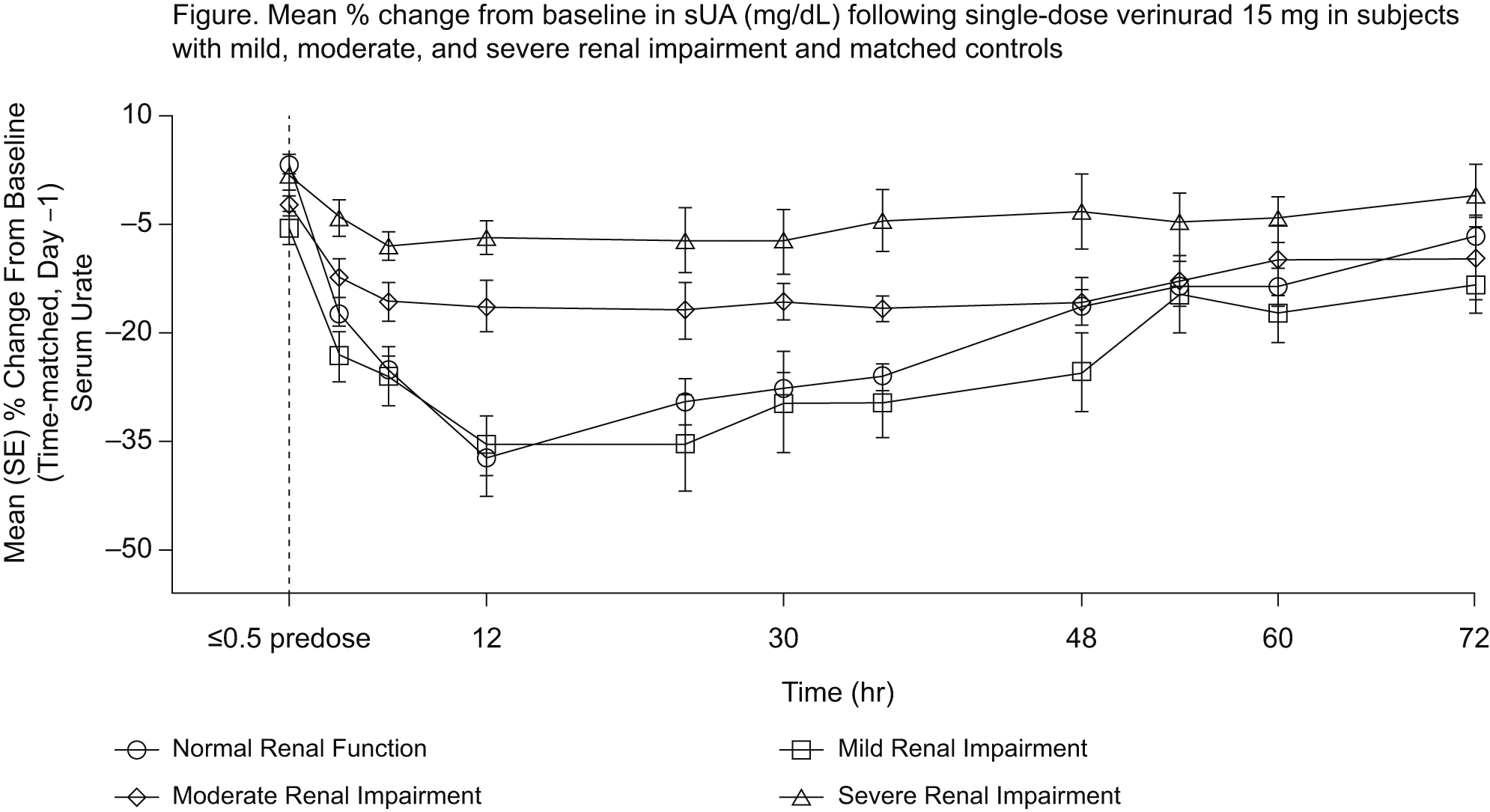
To cite this abstract in AMA style:
Smith WB, Hall J, Berg J, Kazimir M, Yamamoto A, Lee C, Walker S, Marbury TC. Pharmacodynamic and Pharmacokinetic Study of Verinurad in Adult Male Subjects with Mild, Moderate, and Severe Renal Impairment: A Phase 1, Open-Label Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/pharmacodynamic-and-pharmacokinetic-study-of-verinurad-in-adult-male-subjects-with-mild-moderate-and-severe-renal-impairment-a-phase-1-open-label-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacodynamic-and-pharmacokinetic-study-of-verinurad-in-adult-male-subjects-with-mild-moderate-and-severe-renal-impairment-a-phase-1-open-label-study/
