Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: VEXAS syndrome is an adult-onset, X-linked, life-threatening, autoinflammatory and hematological disease caused by somatic mutation in UBA1 gene. Our study aims at uncovering pathophysiology and mechanisms of clonal dominance in VEXAS.
Methods: Nine VEXAS patients (p.Met41 >Thr; p.Met41 >Val; p.Met41 >Leu; c.118-1 G >C) were recruited from our Unit. To introduce UBA1 mutations and develop VEXAS mouse models, base editing technologies were adopted in healthy human HSPCs. Bone marrow (BM) and peripheral blood (PB) cells from patients and mouse xenografts were analyzed by single cell RNA sequencing (scRNA-seq).
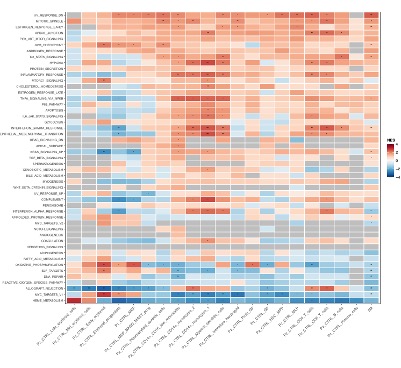
Results: UBA1mut cells from patients were dominant ( >0.85 VAF) in HSPCs and myeloid cells. Conversely, VAF was below 0.05 in T and B cells, supporting a myeloid skewing of mutant HSPCs. Single-cell RNA-seq of bone marrow mononuclear cells (BMMNCs) identified specific HSPC subpopulations in VEXAS patients and revealed pervasive activation of inflammatory signatures and apoptotic responses paralleled by downregulation of cell cycle associated categories in several progenitor and differentiated compartments (Fig. 1). Transcriptional responses indicative of ineffective erythropoiesis were upregulated in VEXAS patients compared to healthy controls, specifically in p.Met41 >Thr individuals.
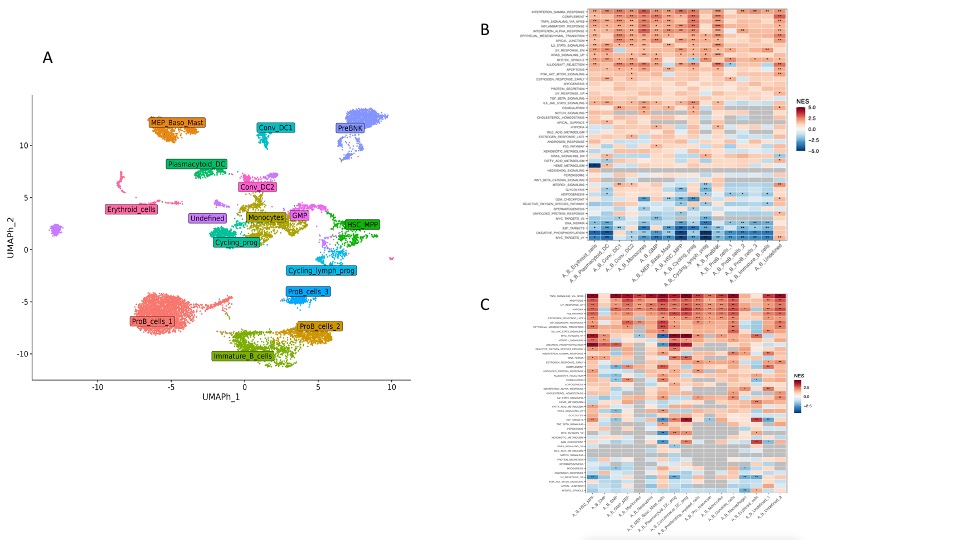
In vivo models of VEXAS syndrome generated by gene editing recapitulated patients’ hematopoiesis and pathophysiology in vitro and in vivo. UBA1 mutations were installed at VAF >0.9 in HSPCs and generated a myeloid bias in vitro and in vivo. In line with evidence in patients, myeloid cells and HSPCs were mostly UBA1mut, while differentiated lymphoid cells were only UBA1wt. scRNA-seq on the human graft from UBA1mut mice revealed pervasive upregulation of inflammatory and apoptotic responses, and lower propensity to engage cell cycle across all hematopoietic subpopulations, particularly of myeloid origin (Fig. 2 A-C). Primitive human HSCs from UBA1mut mice showed early activation of inflammatory responses, premature aging, and transcriptional imprinting toward myelopoiesis. scRNA-seq on the bystander murine cells in UBA1mut mice revealed potent activation of NFkB-mediated inflammatory signatures and apoptosis stimulation. These data, together with competitive transplantation experiments in the mouse model, suggest a mechanism by which the VEXAS clone becomes dominant by poisoning wild-type HSPCs rather than expanding itself.
Conclusion: The in vivo model faithfully recapitulates hematopoiesis of VEXAS patients and highlights a novel mechanism governing clonal dominance that underlies VEXAS pathogenesis.
A. Uniform Manifold Approximation and Projection (UMAP) plot of human CD45+ cells, enriched in the CD34+ HSPC fraction, from the BM of mice transplanted with UBA1mut or UBA1wt HSPCs. Clusters and associated cell types are indicated by name and colors.
B. Gene set enrichment analysis (GSEA) against Hallmark categories showing the transcriptomic profile of sorted human BM populations (panel A) in UBA1mut versus UBA1wt xenografts; NES, normalized enrichment score.
C. Gene set enrichment analysis (GSEA) against Hallmark categories showing the transcriptomic profile of sorted murine BM populations in UBA1mut versus UBA1wt xenografts; NES, normalized enrichment score
To cite this abstract in AMA style:
Campochiaro C, raffaella M, Fiumara M, Tomelleri A, Diral E, Stefanoni D, Varesi A, Weber A, Alfieri R, Albano L, Panigada M, Cantoni E, Canarutto D, Basso-Ricci L, Quaranta P, D’Alessandro A, Bergonzi G, Matucci-Cerinic M, Di Micco R, Aiuti A, Ciceri F, Merelli I, Dagna L, Scala S, Cenci S, Naldini L, Ferrari S, Cavalli G. Pervasive Inflammation Poisons Hematopoiesis and Drives Clonal Dominance in VEXAS Syndrome [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/pervasive-inflammation-poisons-hematopoiesis-and-drives-clonal-dominance-in-vexas-syndrome/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pervasive-inflammation-poisons-hematopoiesis-and-drives-clonal-dominance-in-vexas-syndrome/