Session Information
Date: Monday, October 27, 2025
Title: (1248–1271) Patient Outcomes, Preferences, & Attitudes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus has profound impact on physical, social, and emotional well-being. Health-related quality of life (HRQOL) measures are increasingly incorporated into lupus research and the US Food and Drug Administration has called for patient-reported outcomes (PROs) to be key endpoints in trials. This study sought to explore which HRQOL instruments are viewed by lupus patients as most relevant and reflective of their lived experience.
Methods: Five 90-minute focus groups were conducted, each with 4-6 participants with SLE. Participants were asked to independently complete six commonly used PRO instruments before the session: The Functional Assessment of Chronic Illness Therapy – Fatigue Scale (FACIT-Fatigue), the Lupus Foundation of America Rapid Evaluation of Activity in Lupus (LFA-REAL™), RAND 36-Item Short Form Instrument (SF-36), the Lupus-Quality of Life (Lupus-QoL) Questionnaire, Patients Global Impression of Severity (PGIS-Lupus), and Patients Global Impression of Change (PGIC-Lupus). Time to completion was recorded and written comments were encouraged. A trained moderator guided participants through open-ended discussions exploring instrument ease of use, clarity, relevance, comprehensiveness, and acceptability. Sessions were audio-recorded, transcribed verbatim, and thematically analyzed using an inductive approach.
Results: There were 24 participants aged 29-65 years (33% African American, 13% American Indian, 8% Latinx, and 46% European descent). Demographics are summarized in Table 1. Interrater reliability for qualitative coding was high. Table 2 summarizes the instrument characteristics, completion times, and scores. Most instruments utilized a 4 week recall period; FACIT-Fatigue used 7 days. About 1/3 of participants had difficulty recalling and averaging symptoms over the timespan due to disease variability; others valued the structured reflection. Participants suggested adding items on cognitive dysfunction, headaches, and depression, which they felt were underrepresented and impactful. Difficulty distinguishing lupus-related from comorbid symptoms was a concern, especially with the Lupus QoL, LFA-REAL™, PGIS, and PGIC. The lupus-specific tools and the FACIT-Fatigue were viewed as more relevant. Lupus QoL was preferred by 78% of the participants, alone or combined with the LFA-REAL™ or FACIT-Fatigue, citing its lupus-specific focus, clarity, and coverage of daily function.
Conclusion: This qualitative study provides insights from SLE patients into how well commonly used PRO measures capture their lived experience and symptom burden. The findings may help to determine whether outcome assessments align with priorities and challenges important to patients living with lupus.
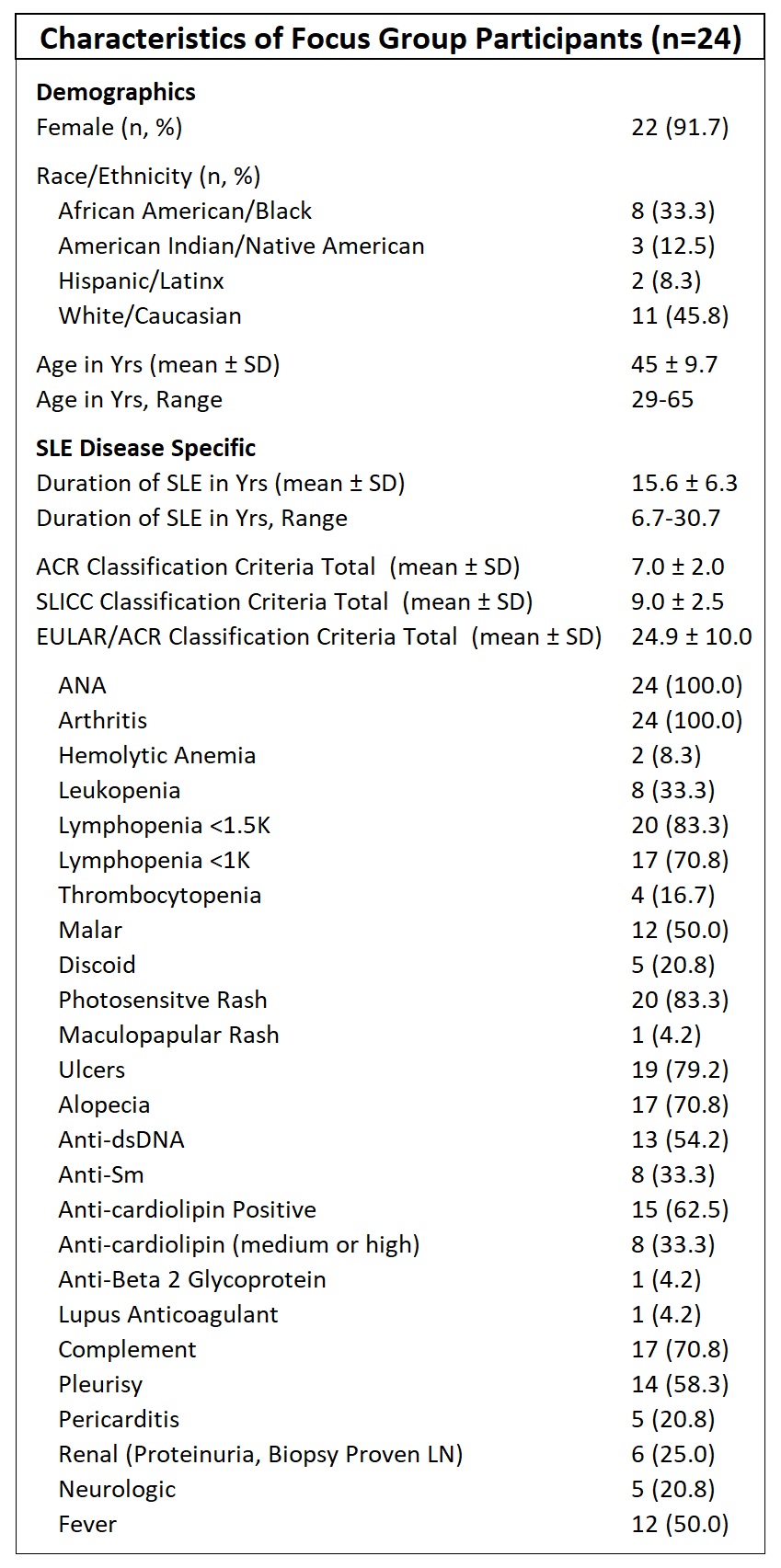 Table 1. Patient Characteristics. Demographic and SLE disease specific information. All patients met the American College of Rheumatology (ACR)-1997 classification criteria, the Systemic Lupus International Collaborating Clinics (SLICC)-2012 classification criteria, and the 2019 EULAR/ACR Classification Criteria.
Table 1. Patient Characteristics. Demographic and SLE disease specific information. All patients met the American College of Rheumatology (ACR)-1997 classification criteria, the Systemic Lupus International Collaborating Clinics (SLICC)-2012 classification criteria, and the 2019 EULAR/ACR Classification Criteria.
.jpg) Table 2. Health Related Quality of Life Instruments. Details the characteristics of each quality of life questionnaire used and presents our participants’ data (completion times, overall and comparable subscale scores) organized to facilitate cross-instrument comparison. Notably, while each individual item on the LFA-REAL™ has a maximum score of 100 millimeters, the total possible score for the entire instrument is 700 millimeters. For the purpose of comparing Pain and Appearance domains with other questionnaires, we combined the LFA-REAL™ scores for Joint Pain and Muscle Pain/Aches into a composite “Pain” score, and similarly combined Rash and Hair Loss scores into a composite “Appearance” score.
Table 2. Health Related Quality of Life Instruments. Details the characteristics of each quality of life questionnaire used and presents our participants’ data (completion times, overall and comparable subscale scores) organized to facilitate cross-instrument comparison. Notably, while each individual item on the LFA-REAL™ has a maximum score of 100 millimeters, the total possible score for the entire instrument is 700 millimeters. For the purpose of comparing Pain and Appearance domains with other questionnaires, we combined the LFA-REAL™ scores for Joint Pain and Muscle Pain/Aches into a composite “Pain” score, and similarly combined Rash and Hair Loss scores into a composite “Appearance” score.
To cite this abstract in AMA style:
Arriens C, Carthen F, Cammarata-Mouchtouris A, James J, Merrill J, Ogunsanya M. Perspectives of Lupus Patients on Quality of Life Measures: A Qualitative Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/perspectives-of-lupus-patients-on-quality-of-life-measures-a-qualitative-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/perspectives-of-lupus-patients-on-quality-of-life-measures-a-qualitative-study/
