Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Ankylosing spondylitis (AS) and psoriatic arthritis (PsA) treatment includes biologics, prescribed on a long-term basis. As persistence data from real-world practice are limited, the current study analyzes persistence with etanercept treatment for up to 5 years in AS or PsA patients in Germany.
Methods: Patients diagnosed with AS or PsA with a first etanercept bio-original (ETNBo) prescription recorded between 1 January 2008 and 31 December 2012 were retrospectively identified in German Statutory health insurance claims data (ICD-10 code M45 and L40.5, respectively) and followed until 31 December 2015 or until the end of their record. The index date was defined as the first ETNBo prescription after an etanercept-free period of ≥12 months.
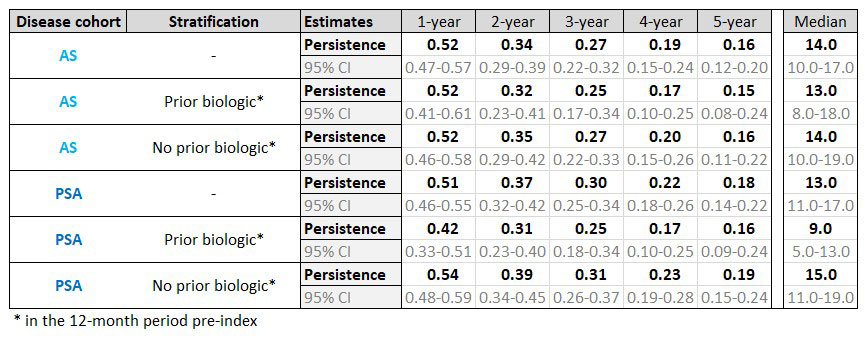
Treatment persistence was estimated as days between index date and discontinuation, defined as a gap of ≥90 days after last etanercept prescription. Analyses were also stratified by whether or not the patients were bio-naive, i.e. having no record of initiating a biologic in the 12 months before index. Persistence was evaluated using Kaplan-Meier analysis. Differences between strata were compared using a log-rank test.
Results: We identified 340 patients diagnosed with AS (mean age at index: 44 years [SD 12], 64% male) and 415 with PsA (49 years [SD 12], 51% male). Most patients were considered bio-naïve (AS: 72%; PsA: 73%).
The median etanercept persistence for AS was 14 months (95%CI: 10–17; IQR: 5–40) and for PsA 13 months (95%CI: 11–17; IQR: 4–43). Persistence for AS ranged from 52% (95%CI: 47%–57%) at 1 year to 16% (95%CI: 12%–20%) at 5 years, and for PsA from 51% (95%CI: 46%–55%) at 1 year to 18% (95%CI: 14%–22%) at 5 years.
For AS, the median etanercept persistence was similar between bio-naïve patients (14 months [95%CI: 10–19) and those with biologic use in the 12 months prior to etanercept (13 months [95%CI: 8–18]) (log-rank test, p-value=0.528). For PsA, median persistence in bio-naïve patients was 15 months (95%CI: 11–19) versus those receiving a biologic in the 12 months pre-index (9 months [95%CI: 5–13]) (log-rank test, p-value=0.065).
Conclusion: This is one of the longest analyses on the real-world persistence of a biologic in AS and PsA. Among new etanercept users in AS and PsA, a quarter of patients were persistent with etanercept for more than 40 months. These results are similar to recent reports from analysis of US claims data1,2; however future research should explore clinical factors influencing persistence over such long time periods.
References
- Walsh JA, Adejoro O, Chastek B, Palmer JB, Hur P. Treatment Patterns Among Patients with Psoriatic Arthritis Treated with a Biologic in the United States: Descriptive Analyses from an Administrative Claims Database. J Manag Care Spec Pharm. March 2018:1-11. doi:10.18553/jmcp.2018.17388
- Hunter T, Schroeder K, Sandoval D, Deodhar A. Persistence, Discontinuation, and Switching Patterns of Newly Initiated TNF Inhibitor Therapy in Ankylosing Spondylitis Patients in the United States. Rheumatol Ther. 2019;6(2):207-215. doi:10.1007/s40744-019-0148-4
To cite this abstract in AMA style:
Baraliakos X, Poddubnyy D, Behrens F, Curiale C, Tarallo M, Hernandez Daly A, Behmer O, Cappelleri J, Hudson N, Gray C. Persistence with Etanercept in Patients with Ankylosing Spondylitis or Psoriatic Arthritis in Germany: A Real-World Analysis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/persistence-with-etanercept-in-patients-with-ankylosing-spondylitis-or-psoriatic-arthritis-in-germany-a-real-world-analysis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistence-with-etanercept-in-patients-with-ankylosing-spondylitis-or-psoriatic-arthritis-in-germany-a-real-world-analysis/