Session Information
Date: Saturday, November 16, 2024
Title: Abstracts: RA – Diagnosis, Manifestations, & Outcomes I: Breathe: RA-ILD
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is an extra-articular manifestation of RA that causes substantial morbidity and mortality. Despite the paucity of known risk factors, there are a number of serum biomarkers that could predict risk. We recently derived a genetic risk score (GRS) for RA-ILD (Wheeler et al., Rheum 2024). We sought to evaluate the discriminative ability of clinical RA-ILD risk factors in combination with this GRS and other peripheral biomarker signatures.
Methods: This was a cross-sectional study of participants within the Veteran’s Affairs Rheumatoid Arthritis registry, a multicenter, prospective cohort of U.S. Veterans with RA. Demographic and clinical data were collected at enrollment. ILD was validated through systematic medical record review of provider diagnosis, imaging, and biopsy findings. ILD status was considered at the time of enrollment, excluding participants who developed RA-ILD more than 2 years after enrollment or with indeterminate ILD. Banked DNA was used for genotyping and serum and plasma collected at enrollment used for biomarker assessment. The GRS included the weighting of 5 SNPs, as previously described (Wheeler et al., Rheum 2024). Sixty-three additional biomarkers were selected based on published associations with RA and/or RA-ILD, encompassing autoantibodies, cytokines/chemokines, adipokines, alarmins, and metalloproteinases. Biomarkers were log-transformed and standardized for analyses. Principal component analysis (PCA) with orthogonal varimax rotation was performed and principal components (PCs) were selected based on eigenvalue >1 and cumulative variance (England et al., ACR Convergence 2021). Biomarkers with loadings >0.3 were considered meaningful. Logistic regression models were compared between clinical factors (age, sex, race, smoking status, disease activity) with and without PCs and GRS using the area under the receiver operating characteristic curve (AUC) and nested likelihood ratio test.
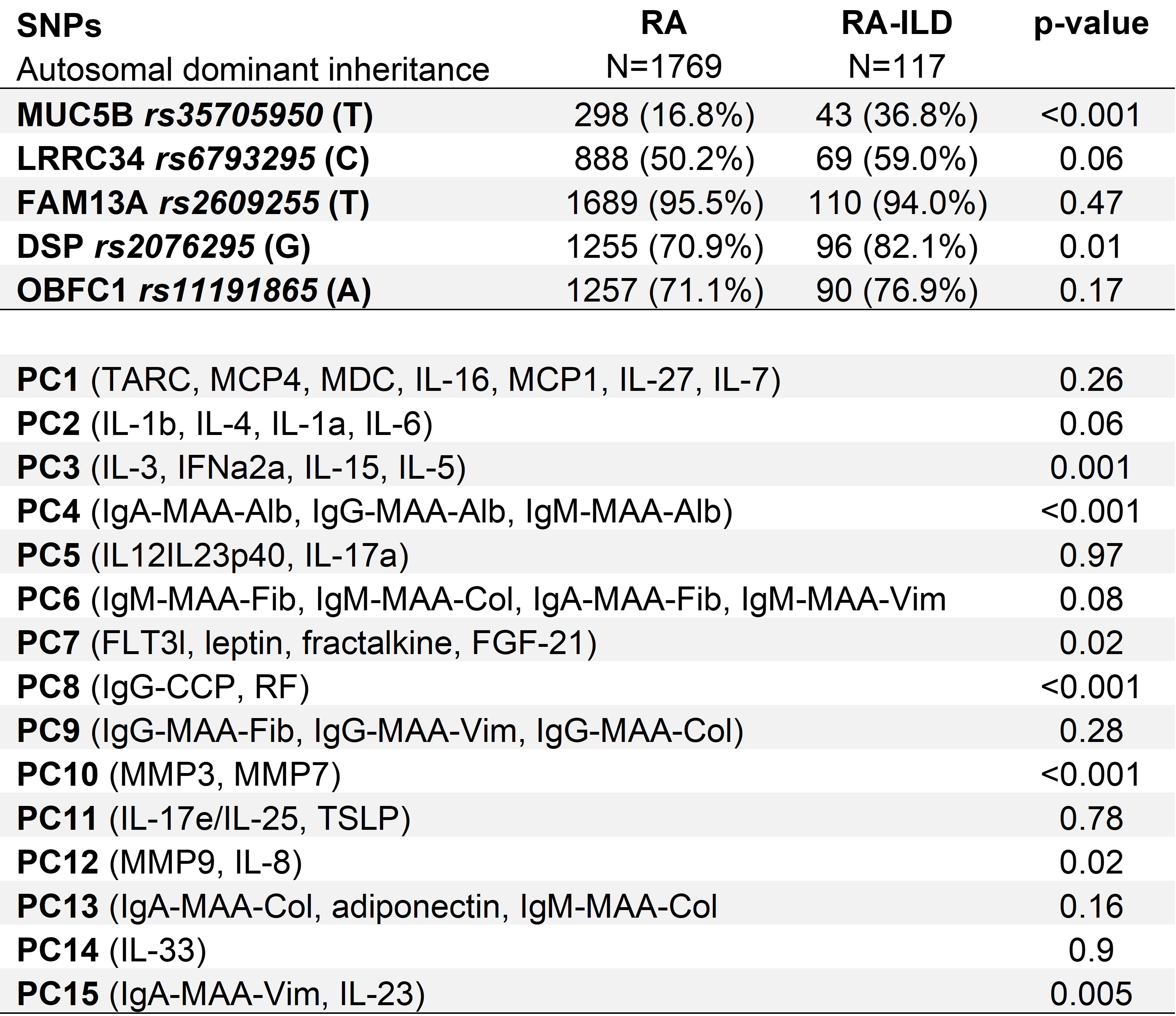
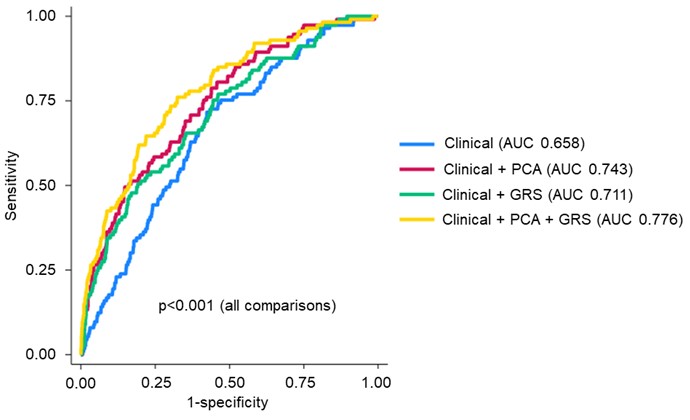
Results: We studied 1,886 RA participants (88.7% male, mean age 64), of whom 117 had RA-ILD. Variant allele frequencies for components of the GRS, assuming autosomal dominant inheritance, are shown in Table 1. PCA yielded 15 PCs, of which 7 were individually associated with RA-ILD (Table 1). Clinical factors alone discriminated RA-ILD with an AUC of 0.658 (95% confidence interval [CI] 0.610-0.706). The addition of either the biomarker PCs (AUC 0.743, 95% CI 0.696-0.789) or the GRS (AUC 0.711, 95% CI 0.661-0.761) significantly improved discrimination (p< 0.001). The combination of clinical factors, biomarker PCs, and the GRS (AUC 0.776, 95% CI 0.732-0.821) was the top performing model (p< 0.001; Figure 1).
Conclusion: The addition of peripheral biomarker signatures and a GRS to clinical risk factors improves the ability to identify RA-ILD among patients with RA. These findings demonstrate the potential for leveraging both genetic and protein biomarkers to offer personalized approaches to inform RA-ILD risk stratification. This study highlights the need for continued analysis and employment of novel biomarkers for RA-ILD identification and prediction.
All PCs with p<0.05 demonstrated higher PC scores in RA-ILD.
Abbreviations: rheumatoid arthritis (RA), rheumatoid arthritis-associated interstitial lung disease (RA-ILD), malondialdehyde-acetaldehyde adducts (MAA), immunoglobulin G (IgG), immunoglobulin M (IgM), immunoglobulin A (IgA), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL), IL_1RA (interleukin_1 receptor antagonist), monocyte chemoattractant protein (MCP), macrophage-derived chemokine (MDC), macrophage inflammatory protein (MIP), thymus and activation regulated chemokine (TARC), tumor necrosis factor (TNF), vascular endothelial growth factor (VEGF), cyclic citrullinated peptide (CCP), rheumatoid factor (RF), matrix metalloproteinase (MMP), fibroblast growth factor (FGF), Fms-like tyrosine kinase 3 ligand (FLT3l), granulocyte colony stimulating factor (G-CSF), IFN (interferon), thymic stromal lymphopoietin (TSLP)
P value by nested likelihood ratio test. Clinical factors include age, sex, race, smoking status, and disease activity score with 28-joint count (DAS28). PCA models include all PCs.
Abbreviations: area under the receiver operating characteristic curve (AUC), principal component analysis (PCA), genetic risk score (GRS)
To cite this abstract in AMA style:
Wheeler A, Riley T, Baker J, Yang Y, Roul P, Wysham K, Cannon g, Kunkel G, Kerr G, Ascherman D, Monach P, Reimold A, Poole J, Merriman T, Mikuls T, England B. Peripheral Biomarker Signatures and a Genetic Risk Score Improve the Identification of RA-ILD Beyond Clinical Risk Factors [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/peripheral-biomarker-signatures-and-a-genetic-risk-score-improve-the-identification-of-ra-ild-beyond-clinical-risk-factors/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/peripheral-biomarker-signatures-and-a-genetic-risk-score-improve-the-identification-of-ra-ild-beyond-clinical-risk-factors/