Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Revised CRISS has been proposed an approvable outcome measure in early diffuse cutaneous SSc (dcSSc)1. The index is undergoing the FDA review as part of the Drug Development Tool Clinical Outcomes Assessments Qualification Program. The Revised CRISS is a two-step process wherein trial patients are determined to have improved (responder) or not improved (non-responder). The first step of the Revised CRISS accounts for worsening or incident cases of any internal organ involvement. If the patient meets any of the Step 1 criteria, their percentage change is 0%, and they are considered a non-responder in a trial. Step 2 includes 5 core set measures. The FVC% is evaluated to see if the change is at least 5% The other 4 core set measures (mRSS, HAQ-DI, Patient (PGA) and Clinician global assessments (CGA)) are evaluated for change of at least 25% from baseline. A responder is a patient who has relative improvement on at least 2 of the 5 core set measures without worsening on more than 1 core set measure. The improvement or worsening must be at least 25% relative change from the baseline for 4 core set measures (or ≥5% for FVC%). Our objective was to assess the performance of Revised CRISS in a phase 3 clinical trial of lenabasum in early dcSSc at week 522.
Methods: The Phase 3 trial was not able to discriminate the active medication vs. placebo and the data was pooled together. Percentages of revised CRISS and corresponding group differences (95% CI) were reported for each factor we explored. Multiple imputation was used for missing data. The imputation model includes five core set measures and three non-missing variables: 1) treatment; 2) indicator of meeting the ACR CRISS step 1; 3) visit weeks. All Results were pooled from each imputed dataset using the Rubin’s rule.
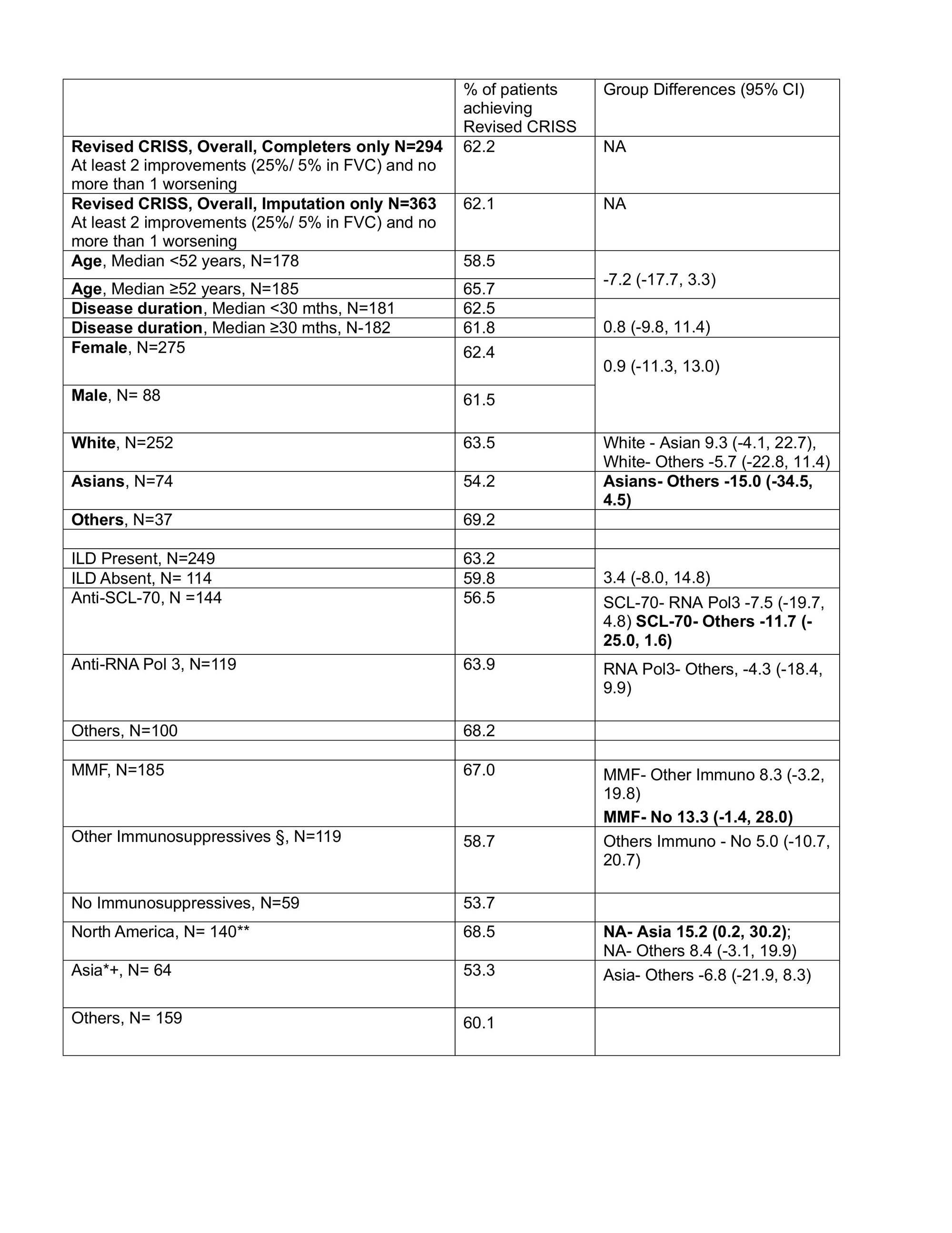
Results: Of 363 patients, 294 (81.0%) are completers, among which 9 (3.1%) met Step 1 (internal organ involvement). The overall response in the Revised CRISS was 62.1% in the imputed dataset. The different baseline factors influenced the response in the Revised CRISS (Table). Using a cutoff of 10% difference between the baseline factors (Table), the variables associated with lower Revised CRISS responses were being an Asian/ living in Asia, anti-SCL-70 antibody, and no background immunosuppressive therapy. Patients who lived in Asia had both lower prevalence of RNA pol3 + (23.4% in Asia vs. 47.9% in North America and 23.3% in Europe), and lower utilization of MMF (23.4% in Asia vs. 74.3% in North America and 41.5% in Europe).
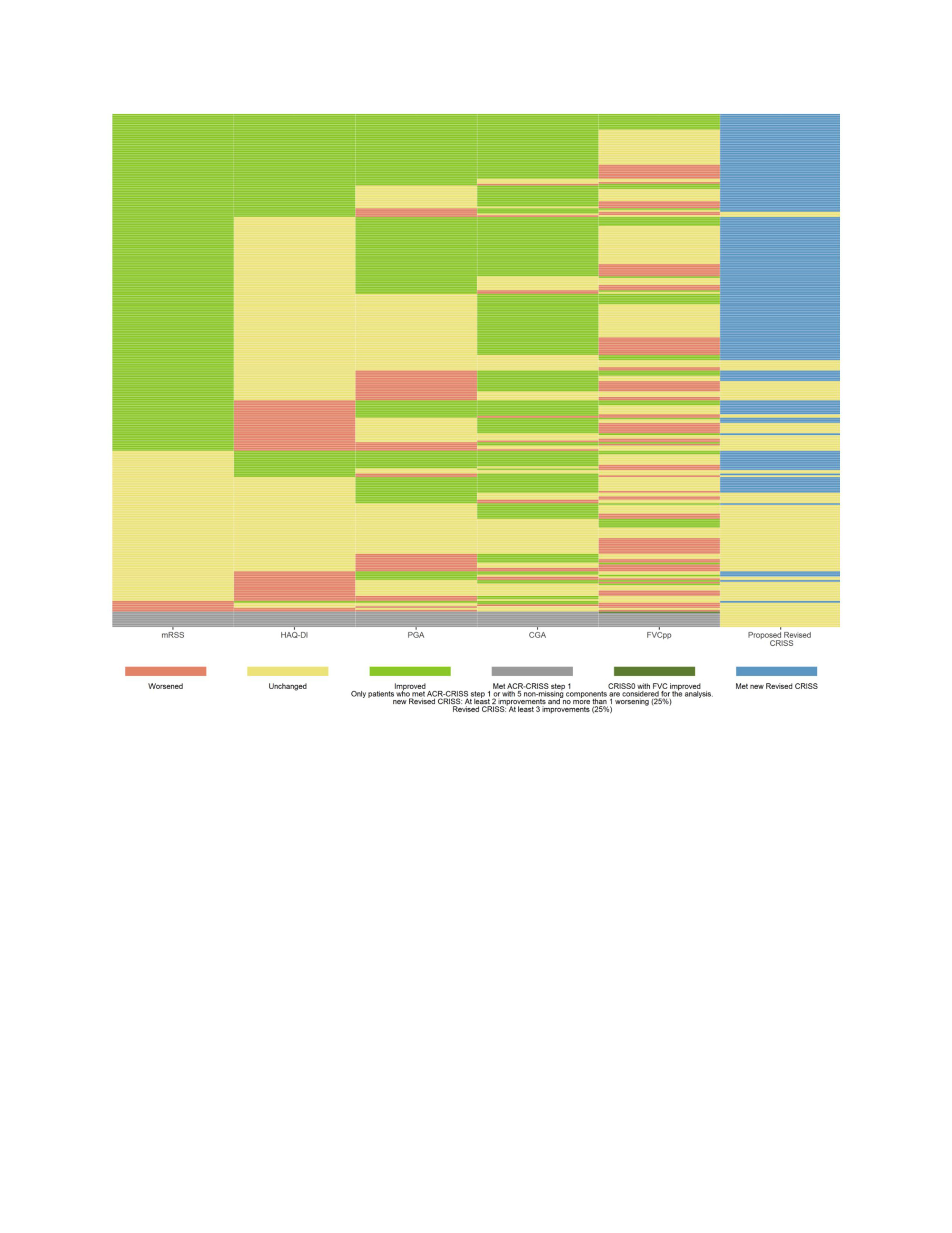
The heatmap (Figure) visualizes the performance of the individual 5 core set measures and the Revised CRISS. There were large improvements in the mRSS followed by CGA that drove a high response in the RCT.
Conclusion: We propose a new preliminary definition of the Revised CRISS that is undergoing the FDA Qualification. The mRSS and CGA drove the Revised CRISS in this RCT, and the future trials should enrich for more severe skin involvement (progressive skin phenotype and less regressive disease) to limit improvement in mRSS (as part of the natural history). In addition, stratification by autoantibodies and background immunosuppressives may balance the Revised CRISS response. 1Khanna D. Ann Rheum Dis 2021 2Spiera R, et al Arthritis Rheumatol 2023
To cite this abstract in AMA style:
Khanna D, Denton C, Kuwana M, Furst D, Huang S, White B, Spiera R. Performance of the Revised CRISS in a Phase 3 Trial of Early Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/performance-of-the-revised-criss-in-a-phase-3-trial-of-early-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/performance-of-the-revised-criss-in-a-phase-3-trial-of-early-systemic-sclerosis/