Session Information
Date: Monday, November 18, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) is a current standard for evaluating rashes in Dermatomyositis (DM). However, there is increasing emphasis on patient-centric outcome measures that incorporate the patient’s perspective of the disease. Moreover, with increasing telemedicine visits, a patient self-assessment of DM rashes is valuable for remote monitoring. We sought to validate self-assessment tools for rash in DM patients.
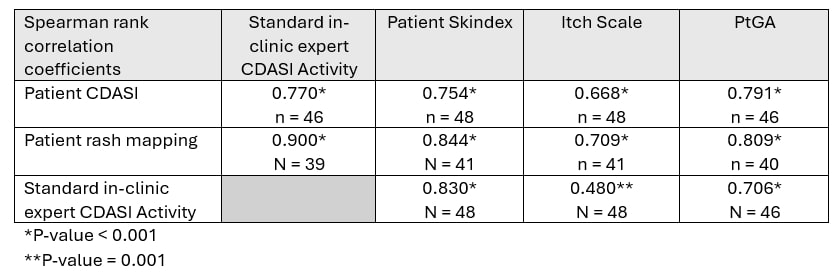
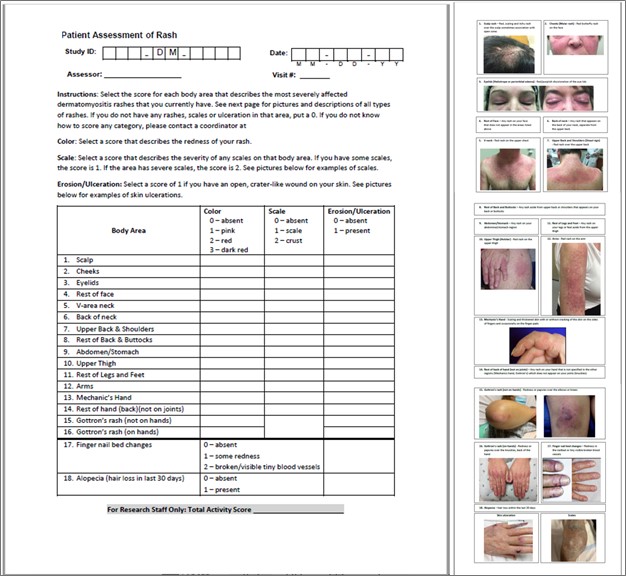
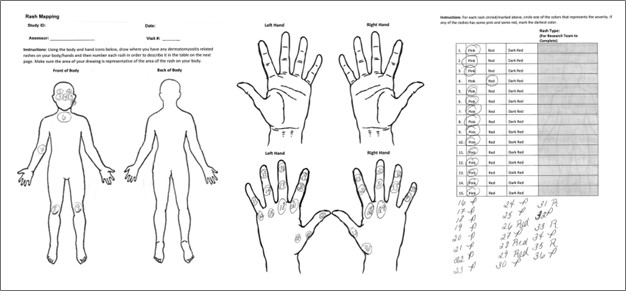
Methods: Two tools were utilized: “Patient Cutaneous Dermatomyositis Disease Area and Severity Index (Patient CDASI),” a simplified version of the CDASI modified for the patients, and “Rash mapping.” Rash mapping involves front and back body icons, where patients mark the areas of rashes and rate their severity based on redness. Using Spearman rank correlation coefficients, we validated these tools against the standard in-clinic myositis expert-evaluated CDASI and standard patient-reported outcomes (Skindex, Itch Scale, and Patient global assessment of disease activity-PtGA) completed at the same visit. Active DM skin disease, defined by the physician’s visual analog scale (VAS) ≥ 3, was also assessed by myositis experts.
Results: 27 DM patients [81.5% female, 96.3% Caucasian; median (IQR) age 50.0 (38.0 – 61.0) years and a median (IQR) disease duration 38.0 (9.5 – 88.0) months] satisfying the ACR/EULAR 2017 classification criteria with 1-2 clinic visits spaced at least 3 months apart were enrolled. The most common myositis-specific antibody was TIF1-γ (13 patients, 48.1%). Half of the patients had active DM skin disease. The median (IQR) of the standard CDASI activity score was 6.0 (0.0 – 17.0), and the median (IQR) Skindex score was 28.0 (4.0 – 45.0). The Spearman rank correlation coefficients for patient CDASI with the standard CDASI, Skindex, Itch Scale, and PtGA were 0.77, 0.75, 0.67, and 0.79, respectively (p< 0.01 for all). The correlation coefficients for rash mapping with the standard CDASI, Skindex, Itch Scale, and PtGA were 0.84, 0.87, 0.75, and 0.83, respectively (p< 0.01 for all).
Conclusion: Our findings demonstrate the favorable validity of both patient CDASI and patient rash mapping tools for DM patients to self-evaluate their rashes.
To cite this abstract in AMA style:
Pongtarakulpanit N, Chandra T, keret s, Gkiaouraki E, Liarski V, Ascherman D, Mogahadam S, Oddis C, Aggarwal R. Performance of the Patient Self-Assessment Tools for Cutaneous Dermatomyositis: Patient CDASI and Rash Mapping [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/performance-of-the-patient-self-assessment-tools-for-cutaneous-dermatomyositis-patient-cdasi-and-rash-mapping/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/performance-of-the-patient-self-assessment-tools-for-cutaneous-dermatomyositis-patient-cdasi-and-rash-mapping/