Session Information
Date: Saturday, November 6, 2021
Title: Sjögren's Syndrome – Basic & Clinical Science Poster (0296–0322)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Recent randomised controlled trials (RCTs) in primary Sjögren’s syndrome (pSS) failed to show clinical efficacy.1-3 Several RCTs used the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) as primary endpoint, showing large response rates in active treatment and placebo groups.1,3 The ESSDAI consists of some domains which are more sensitive to change and easy to score compared to other domains.4 The objective of this study was to develop the Clinical Trials ESSDAI (ClinTrialsESSDAI), an adjusted ESSDAI score consisting of frequently active and sensitive to change domains, and to compare the performance of the ClinTrialsESSDAI to the Clinical (Clin)ESSDAI and ESSDAI in two RCTs.
Methods: The ASAP-III trial in abatacept (80 pSS patients)1 and TRACTISS trial in rituximab (133 pSS patients)2 were used for development and analyses of the ClinTrialsESSDAI. These trials were selected since ASAP-III included only patients with moderate to high disease activity according to ESSDAI, whereas TRACTISS did not apply this inclusion criterion, leading to higher baseline ESSDAI scores in ASAP-III than in TRACTISS (median 13 vs. 4). Activity of ESSDAI domains at baseline was analysed. The six most frequently active domains were selected for the ClinTrialsESSDAI, with exclusion of the biological domain. This is in line with the ClinESSDAI, which does not include the biological domain, to measure a ‘true’ clinical effect. The ClinTrialsESSDAI score was calculated using existing weights of the ClinESSDAI. Responsiveness of the ClinTrialsESSDAI, ClinESSDAI and ESSDAI was calculated using the standardised response mean (SRM) at the primary endpoint visits: week 24 for ASAP-III, week 48 for TRACTISS. Percentage of patients who reached the minimal clinically important improvement (MCII, decrease of ≥3 points) and low disease activity (LDA, score < 5) was analysed for all three scores.
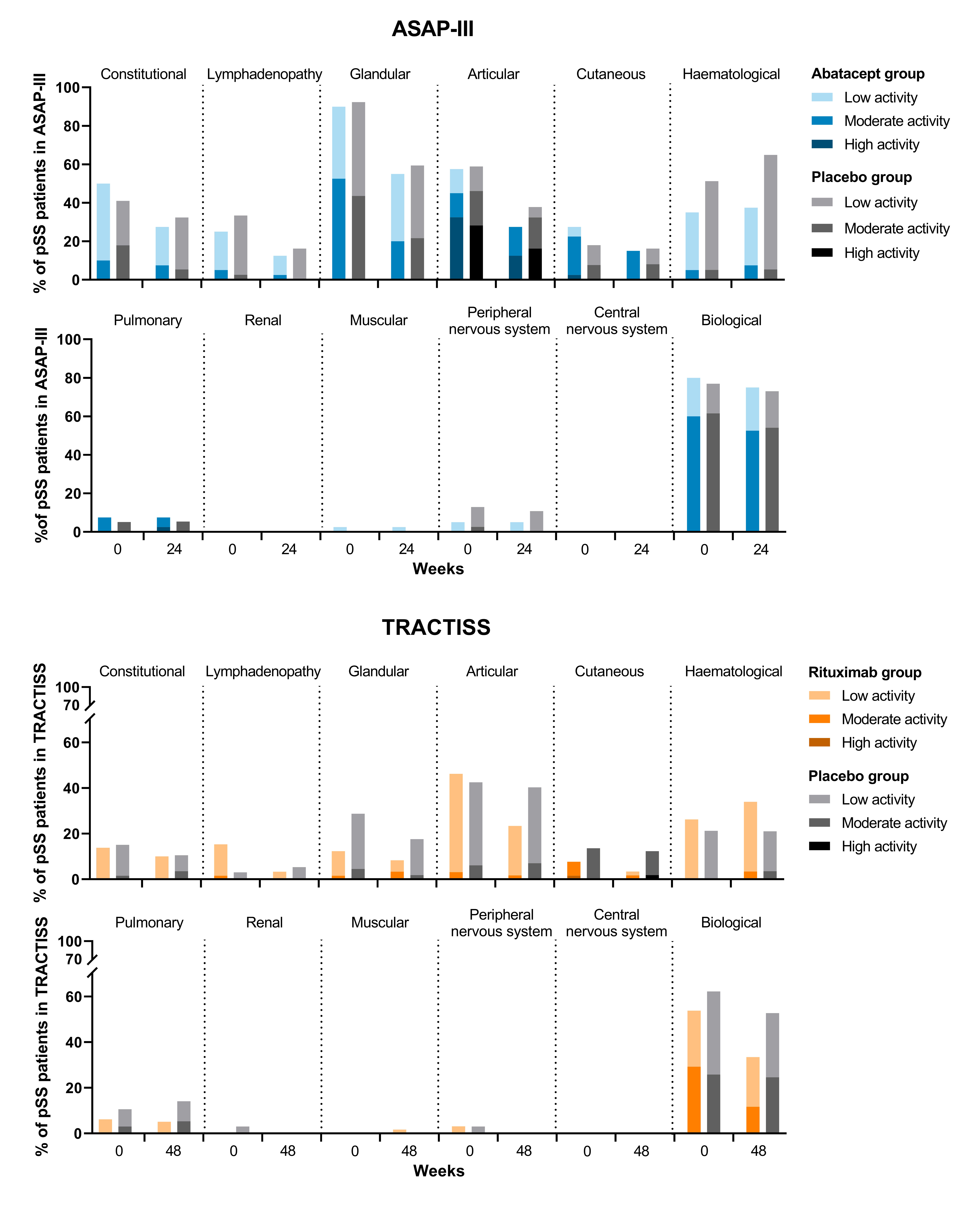
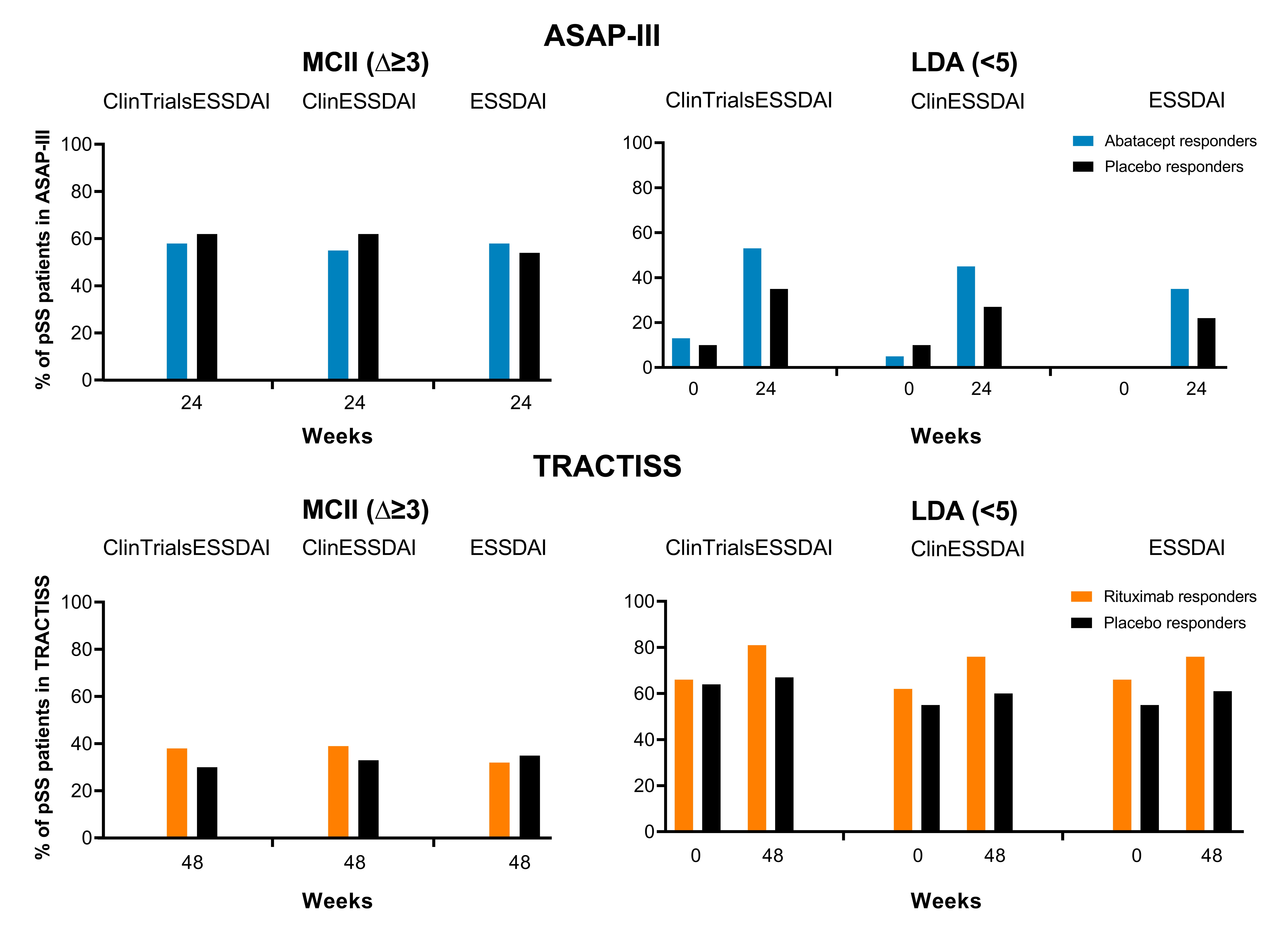
Results: Besides the biological domain, the six most frequently active domains at baseline in the ASAP-III and TRACTISS trial were the glandular (any activity: 91% and 21%, respectively), articular (58% and 44%), haematological (43% and 24%), constitutional (46% and 15%), lymphadenopathy (29% and 9%) and cutaneous (23% and 11%) domain (Figure 1). These domains were selected for the ClinTrialsESSDAI. Responsiveness was similar using any of the three scores at primary endpoint visits of the ASAP-III and TRACTISS trials (Table 1). Discrimination between active treatment and placebo groups based on MCII and LDA responders was also similar using any of the three scores (Figure 2).
Conclusion: The ClinTrialsESSDAI, consisting of six frequently active and sensitive to change domains of the ESSDAI, shows similar responsiveness and discrimination between treatment groups compared to the ClinESSDAI and ESSDAI in the ASAP-III and TRACTISS RCT. Therefore, this ClinTrialsESSDAI will not solve the problem of discrimination and a composite endpoint combining response at multiple clinically relevant items seems more suitable as primary study endpoint.
References
1. Van Nimwegen 2020;9913:1-11
2. Bowman 2017;69:1440-50
3. Baer 2021(doi:218599)
4. De Wolff 2020;38,Suppl 126:283-90
To cite this abstract in AMA style:
de Wolff L, Arends S, Pontarini E, Bombardieri M, Bowman S, Bootsma H. Performance of the Clinical Trials ESSDAI (ClinTrialsESSDAI), an Adjusted ESSDAI Score, in Two Randomised Clinical Trials in Patients with Primary Sjögren’s Syndrome [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/performance-of-the-clinical-trials-essdai-clintrialsessdai-an-adjusted-essdai-score-in-two-randomised-clinical-trials-in-patients-with-primary-sjogrens-syndrome/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/performance-of-the-clinical-trials-essdai-clintrialsessdai-an-adjusted-essdai-score-in-two-randomised-clinical-trials-in-patients-with-primary-sjogrens-syndrome/