Session Information
Date: Monday, October 22, 2018
Title: Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster II: Diagnosis and Prognosis
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Antibodies to peptidylarginine deiminase (PAD) enzymes have been implicated in the pathogenesis of RA. Previous studies have shown that patients with PAD4 antibodies have more erosive joint disease compared to patients who do not. We hypothesized that patients with PAD4 antibodies would have more severe disease and report higher levels of RA symptoms and functional impacts than those without antibodies.
Methods:
Adults with MD-diagnosed RA enrolled in a longitudinal cohort study with serum available from the visit were included; almost all (96%) met 2010 ACR/EULAR RA criteria. Anti-PAD4 antibodies were detected by immunoprecipitation. Independent t-tests and chi-square were used to compare patient and RA characteristics and selected patient reported outcomes (PROs: PROMISTM pain intensity, physical function, fatigue, ability to participate in social roles and activities, anxiety, depression, and sleep disturbance) by antibody status.
Results:
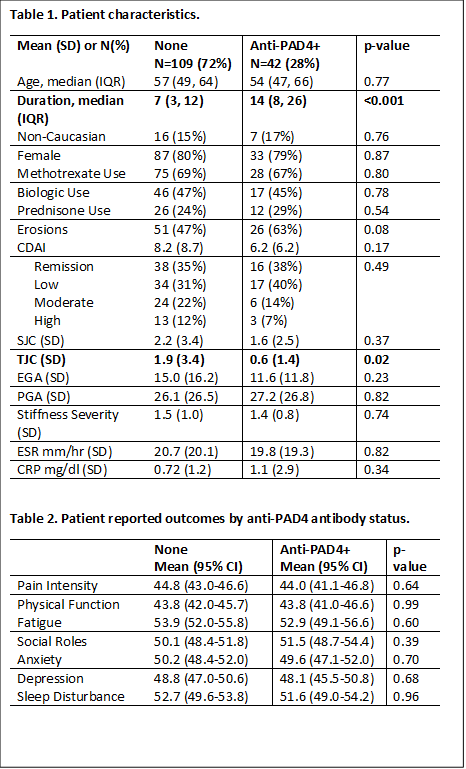
Patients (n=151) had a mean age of 55 +/-13, were mostly female (80%), and white (85%). Most had longstanding RA (11 +/-10 years) with 35% in CDAI remission and 34% with low disease activity. Nearly half (47%) were on a biologic, and 68% were on MTX. Anti-PAD antibodies and PROs were available on 135 patients (89%); baseline characteristics did not differ between those with and without completed PROs. A total of 38 (28%) patients were anti-PAD4+. No significant differences were evident between groups in mean CDAI, SJC, PGA, EGA, stiffness severity, ESR, or CRP (Table 1). Although a higher proportion of anti-PAD4+ patients had erosions, there was no statistically significant difference between groups (Table 1). Mean PROMIS scores (pain intensity, physical function, fatigue, ability to participate in social roles and activities, anxiety, and depression) were in the normal range (i.e., 55-65) except pain intensity and physical function which were lower in both groups (Table 2); however, PRO scores were similar between groups.
Conclusion:
In this well characterized cohort of RA patients, anti-PAD4+ patients had longer disease duration and a slightly lower TJC compared to patients without PAD antibodies. Anti-PAD4+ patients had a trend toward more erosive disease; but PROs were similar between groups. Contrary to expectations, anti-PAD4+ patients did not have clinical evidence of worse symptoms or functional impacts. The lack of congruency between bony damage and disease activity may suggest a difference in pathogenesis between these processes.
 |
To cite this abstract in AMA style:
DiRenzo D, Darrah E, Bartlett SJ, Bingham III CO, Cappelli LC. Peptidylarginine Deiminase-4 Antibodies Are Not Associated with Worse RA Activity, Symptoms or Impacts [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/peptidylarginine-deiminase-4-antibodies-are-not-associated-with-worse-ra-activity-symptoms-or-impacts/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/peptidylarginine-deiminase-4-antibodies-are-not-associated-with-worse-ra-activity-symptoms-or-impacts/
