Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Knee OA is a common disease affecting ~15 million people in the United States. There is an urgent unmet need for additional OA treatments as current treatments provide only short-term pain relief often associated with unwanted side effects and contraindications. Gene therapy using viral vectors has emerged as a novel therapeutic modality with the potential to treat many diseases. PCRX-201 (enekinragene inzadenovec) is a novel high-capacity adenovirus serotype 5 (Ad5) vector carrying a transgene encoding for the inflammation-inducible expression of IL-1 receptor antagonist (IL-1Ra) that is under investigation to treat knee OA via IA injection. This open-label phase 1 trial (NCT04119687) investigated the safety and efficacy of PCRX-201, for which long-term follow-up is ongoing.
Methods: Patients aged 30-80 years (Nf72) with painful OA of the index knee and Kellgren/Lawrence (K/L) grade 2, 3, or 4 were eligible. The first group (n=36) received IA injection of PCRX-201 in the target knee at 1 of 3 doses (low: 1.4E10 genome copies [GC]; middle: 1.4E11 GC; high: 1.4E12 GC); the second group (n=36) was pretreated with IA methylprednisolone 40 mg immediately before PCRX-201 administration at the same doses. WOMAC pain (WOMAC-A) and stiffness (WOMAC-B) subscale scores and Knee Injury and Osteoarthritis Outcome Score Activities of Daily Living (KOOS ADL) were measured up to 156 weeks. Anti-Ad5 neutralizing antibody (NAb) titers in serum were measured at baseline before PCRX-201 administration and up to 52 weeks.
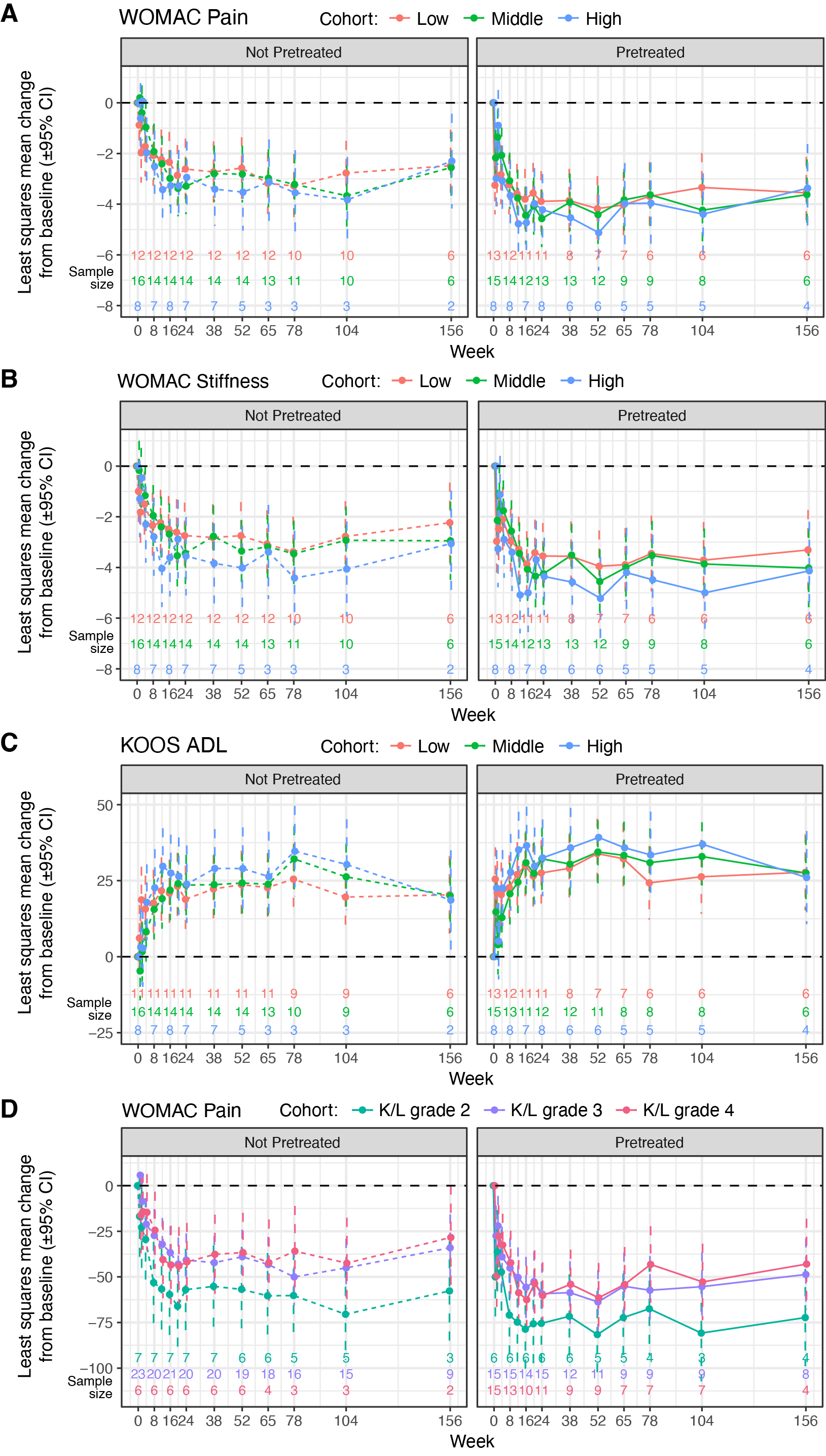
Results: Pain and function improvements were observed at all doses and across both the not pretreated and corticosteroid pretreated groups up to 156 weeks after PCRX-201 IA administration (Figure 1). The pretreated group showed greater improvements for WOMAC-A (range of least squares mean [LSM] improvement from baseline across dose levels, 3.37-3.62 [of 10] points; Figure 1A), WOMAC-B (3.31-4.13 [of 10] points; Figure 1B), and KOOS ADL (Figure 1C) than the not pretreated group. Patients in the pretreated group with K/L grades of 2, 3, and 4 showed LSM reductions from baseline in WOMAC-A, with greater numerical reductions observed in those with K/L grade 2 than those with K/L grades 3 or 4 (Figure 1D). Importantly, preexisting serum NAbs did not affect PCRX-201 efficacy as determined by WOMAC-A pain (Figure 2A) and WOMAC-B stiffness (Figure 2B). The most commonly reported treatment-related adverse event (AE), index joint effusion, occurred less frequently for participants in the pretreated (15/36; 42%) than the not pretreated group (24/36; 67%) (Table 1).
Conclusion: A single IA injection of PCRX-201 with corticosteroid pretreatment in patients with knee OA had an acceptable safety profile and sustained clinical efficacy for up to 156 weeks. Preexisting NAbs did not affect PCRX-201 efficacy or safety at all 3 doses, and corticosteroid pretreatment demonstrated greater improvement in the clinical scores than no pretreatment. These results support the ongoing phase 2 study strategy of PCRX-201 with a 10-fold lower dose (1.4E11 GC) and steroid pretreatment.
.jpg) Figure 1. (A) WOMAC pain scores over time by dose for each cohort. (B) WOMAC stiffness scores over time by dose for each cohort. (C) KOOS ADL scores over time by dose for each cohort. (D) WOMAC pain scores by K/L grade for each cohort.
Figure 1. (A) WOMAC pain scores over time by dose for each cohort. (B) WOMAC stiffness scores over time by dose for each cohort. (C) KOOS ADL scores over time by dose for each cohort. (D) WOMAC pain scores by K/L grade for each cohort.
.jpg) Figure 2. WOMAC pain scores (A) and WOMAC stiffness scores (B) by serum baseline neutralizing antibody status for each cohort.
Figure 2. WOMAC pain scores (A) and WOMAC stiffness scores (B) by serum baseline neutralizing antibody status for each cohort.
To cite this abstract in AMA style:
Cohen S, Conaghan P, Hochberg M, Kivitz A, Kim M, Joy N, Jiang S, DiGiorgi M, Slonin J, Slonin D. PCRX-201 High-Capacity Adenovirus Serotype 5 Gene Therapy Demonstrates Sustained Clinical Efficacy and Safety in Patients With Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/pcrx-201-high-capacity-adenovirus-serotype-5-gene-therapy-demonstrates-sustained-clinical-efficacy-and-safety-in-patients-with-knee-osteoarthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pcrx-201-high-capacity-adenovirus-serotype-5-gene-therapy-demonstrates-sustained-clinical-efficacy-and-safety-in-patients-with-knee-osteoarthritis/