Session Information
Session Type: Abstract Session
Session Time: 11:30AM-11:45AM
Background/Purpose: VEXAS syndrome (Vacuoles, E1 ubiquitin-activating enzyme, X-linked, Autoinflammatory, Somatic) is a systemic disorder characterized by an overlap of hematologic and inflammatory features. Treatment poses a challenge, as most immunomodulators fail to control the disease, resulting in a requirement for moderate to high doses of glucocorticoids (GCs). Pacritinib, an oral inhibitor of IRAK1, JAK2, and ACVR1, is FDA-approved for myelofibrosis with severe thrombocytopenia (platelets < 50 x 109/L) and has emerged as a potential treatment for VEXAS syndrome.
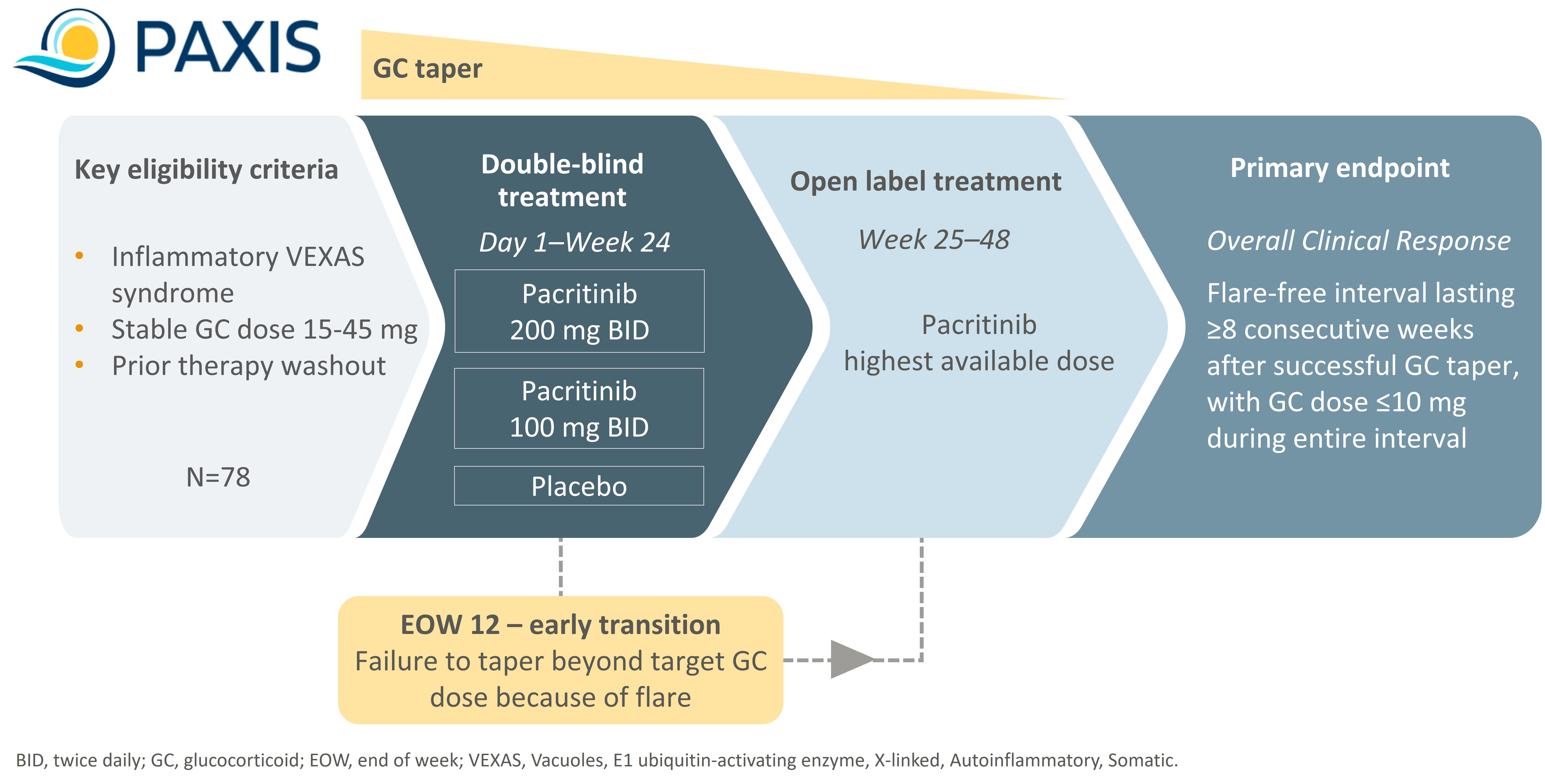
Methods: PAXIS is an international, randomized, multicentre, double-blind, placebo-controlled phase 2 dose finding trial. It is designed to assess pacritinib in adult patients with VEXAS syndrome. Inclusion criteria include documented evidence of past inflammatory involvement and ongoing GCs for ≥4 consecutive weeks at a baseline dose of 15-45 mg/day. Previous exposure to non-GC therapies is allowed, but patients must undergo a washout period. Exclusion criteria include prior allogeneic hematopoietic stem cell transplantation, receiving ≥9 units of red blood cell transfusion in the prior 90 days, concurrent myelodysplastic syndrome requiring antineoplastic treatment, and exposure to any HMAs in the previous 6 months or exposure to more than 4 cycles of HMAs at any time.The study will consist of a screening period, a double-blind period (up to end of week 24), an open-label period (up to end of week 48), and a 30-day post-end of treatment period (Figure 1). Patients (n=78) will be randomized 1:1:1 to receive pacritinib 200 mg twice daily (BID), pacritinib 100 mg BID, or placebo (26 per arm). All patients will follow a fixed GC taper.
Results: The primary endpoint (assessed during the double-blind period) is the proportion of patients who achieve Overall Clinical Response, defined as having a flare-free interval lasting ≥8 weeks following a GC taper, and a GC dose during the interval of ≤10 mg/day. Secondary endpoints include number of flare free days with GC dose < 10 mg/day, hematologic improvement (in platelets and hemoglobin), change in quality-of-life measures, and safety. Exploratory endpoints include change in symptoms based on the VEXAS-symptom assessment form (VEXAS-SAF), change in disease activity measured by the Clinical Global Impression of Change (CGI-C) and the VEXAS-disease activity index (VEXAS-DAI), change in variant allele frequency (VAF) of UBA1 by next generation sequencing (NGS), and improvement in GC toxicity as measured by the glucocorticoid toxicity index (GTI). PAXIS is anticipated to enroll at 40 sites across 8 countries.
Conclusion: The PAXIS study represents a significant step forward in the treatment of VEXAS syndrome. As the first randomized, double-blind placebo-controlled pharmacotherapy trial for this disease, it has the potential to redefine treatment and address unmet needs for this complex, refractory, and sometimes fatal condition.This abstract has been accepted for publication by the European Alliance of Associations for Rheumatology (EULAR). The definitive copyedited version is available online at: www.ard.eular.org
To cite this abstract in AMA style:
Beck D, Heiblig M, Savic S, ferrada M, Mekinian A, Chowdhury O, Hammond D, Weeks L, Gurnari C, Kirino Y, georgin-Lavialle S, Buckley S, harder B, Goble S, Koster M. PAXIS: A Randomized, Double-Blind, Placebo-Controlled, Dose Finding Phase 2 Study (Part 1) Followed by an Open-Label Period (Part 2) to Assess the Efficacy and Safety of Pacritinib in Patients with VEXAS Syndrome [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/paxis-a-randomized-double-blind-placebo-controlled-dose-finding-phase-2-study-part-1-followed-by-an-open-label-period-part-2-to-assess-the-efficacy-and-safety-of-pacritinib-in-patients-with-ve/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/paxis-a-randomized-double-blind-placebo-controlled-dose-finding-phase-2-study-part-1-followed-by-an-open-label-period-part-2-to-assess-the-efficacy-and-safety-of-pacritinib-in-patients-with-ve/