Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Based mainly on results from a randomized clinical trial (RCT) in rheumatoid arthritis (RA) patients (ORAL Surveillance), major regulatory agencies have concluded that JAK inhibitors, including Upadacitinib (UPA), a selective and reversible JAK1 inhibitor, may be associated with an increased risk of serious cardiovascular events and cancer compared with tumor necrosis factor inhibitors (TNFi). In this context, evidence on safety, effectiveness and risk minimization measures in patients treated with UPA in routine clinical practice are of particular interest.
Methods: Observational, retrospective, and multicenter study including patients diagnosed with RA, and spondylarthritis (SpA) (psoriatic arthritis, and axial-SpA), who received UPA for at least 3 months between January 1, 2021, and March 31, 2023. For a comparison of baseline cardiovascular risk (CVR), RA and SpA patients who initiated TNFi treatment during this same period were also analyzed. Baseline demographic and disease characteristics, changes over time in DAS28 or BASDAI, baseline CVR by Systemic Coronary Risk Estimation (SCORE)2 or SCORE2_OP (patients >70 years), concomitant medications, line and persistence of UPA treatment, and adverse events data were collected. Cox regression and Kaplan-Meier curves were used to analyze the UPA persistence, and a general mixed linear model to study the variation of disease activity over time. Chi-squared, t-Student, and U-Mann-Whitney tests were used to compare groups.
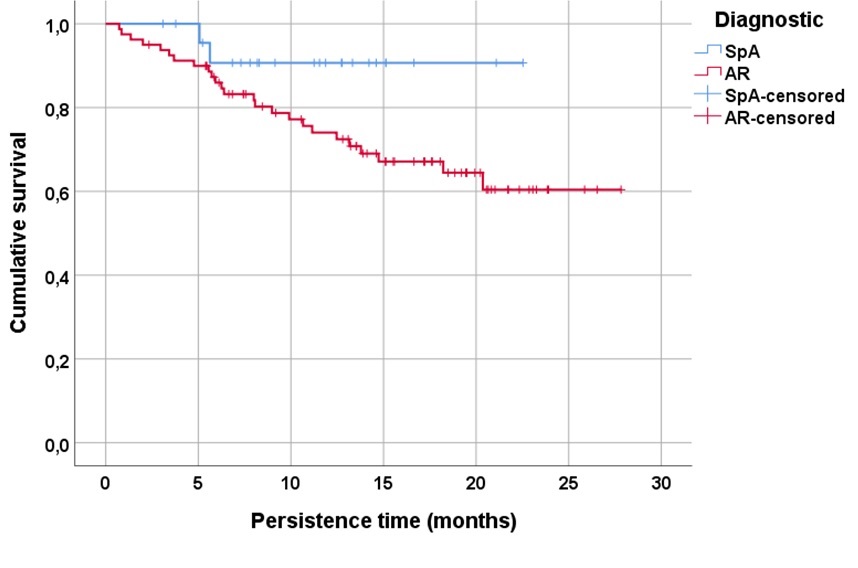
Results: A total of 104 patients treated with UPA, 80 RA (77%) and 24 (23%) SpA, were included. The mean follow-up time was 12.8±6.7 (SD) months. Table 1 shows baseline demographic and disease characteristics, disease activity, and CVR factors, including baseline SCORE2 of patients treated with UPA and TNFi. UPA showed significant effectiveness in controlling RA disease activity by DAS28 during follow-up (p< 0.001). UPA persistence in both RA and SpA patients is shown in Figure 1, with a tendency to a better persistence in SpA (log rank: 2.9, p=0.08). According to the COX regression analysis, the only factor associated with lower UPA persistence was its use in 2nd or higher line (HR 7.1 95%CI 0.98-52.08, p= 0.052). The main reason for discontinuation of UPA was adverse events (n=11, 44%), followed by ineffectiveness (n=8, 32%) and loss to follow-up (n=6, 24%). To examine changes over time in CVR minimization in UPA-treated patients, 109 patients who initiated TNFi during the same period with similar demographic characteristics and clinical activity (Table 1) were analyzed as a comparator. SCORE2, both as a percentage (p=0.47) and categorized (p=0.21), were similar between patients treated with UPA or TNFi (Table 1). However, when SCORE2 was categorized and analyzed by trimester, a significant reduction (p=0.03) was observed in moderate-high risk patients who initiated UPA versus TNFi since April 2022 (Table 2).
Conclusion: UPA shows sustained effectiveness in patients with RA and especially in those with SpA, with a safety profile equivalent to that shown in RCTs. The recommendations of the regulatory authorities to minimize CVR in patients treated with JAKi seem to have been adopted and even anticipated in daily clinical practice.
To cite this abstract in AMA style:
Garcia Dorta A, Hernández Martín A, Rodríguez Regalado C, Peña Montelongo S, Martínez González C, Naveda-González E, González-Dávila E, Diaz-Gonzalez J, Hernández Hernández m. Patterns of Use, Effectiveness, Persistence and Cardiovascular Risk in Patients with Rheumatic Diseases Treated with Upadacitinib in a Real-world Setting. UPAREAL Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/patterns-of-use-effectiveness-persistence-and-cardiovascular-risk-in-patients-with-rheumatic-diseases-treated-with-upadacitinib-in-a-real-world-setting-upareal-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patterns-of-use-effectiveness-persistence-and-cardiovascular-risk-in-patients-with-rheumatic-diseases-treated-with-upadacitinib-in-a-real-world-setting-upareal-study/