Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Although the increasing availability of biologic disease-modifying antirheumatic drugs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) has significantly improved outcomes for patients with Juvenile Idiopathic Arthritis (JIA), a substantial proportion of patients do not respond to an initial therapy, requiring switching to a second bDMARD/tsDMARD. Subsequent treatment decisions for children with continuing disease activity are haphazard, following a “trial and error approach”. The objective of this study is to characterize patterns of bDMARD/tsDMARDswitching among patients with JIA in real-world clinical settings following the initial bDMARD/tsDMARD therapy.
Methods: We conducted a retrospective study of a national administrative claims database in the US (January 2008 to March 2020). Study subjects included children (age < 19 years) who initiated a new bDMARD/tsDMARD therapy, had a medical claim associated with JIA diagnosis, and had been enrolled in the health plans for at least 6 months prior to starting initial bDMARD/tsDMARD therapy. Medications of interest comprised tumor necrosis factor inhibitors (TNFis), abatacept, tocilizumab, anakinra, canakinumab, tofacitinib, ustekinumab, rituximab, and secukinumab.
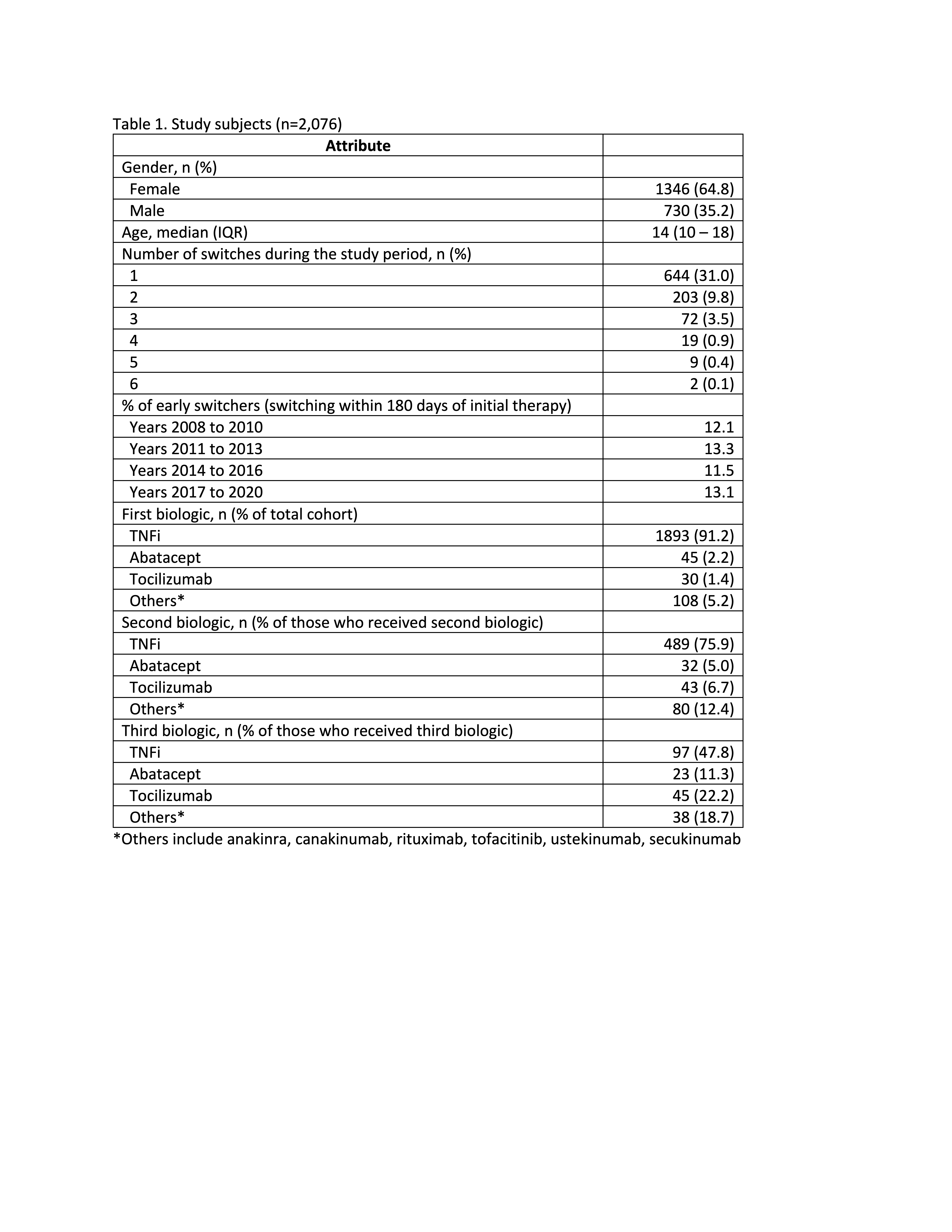
Results: 2,076 eligible children with JIA were prescribed bDMARD/tsDMARD therapy, most of whom started with TNFis (91%) (Table 1). Median follow-up was 699 days following first reported bDMARD/tsDMARD prescription (IQR 289-1503). During follow-up, 644 (31%) switched to a second bDMARD/tsDMARD, most commonly to a second TNFi (76%). A further 203 (9.8%) switched to a third bDMARD/tsDMARD and 30 (1.4%) patients received 4 or more bDMARD/tsDMARD therapies. The median time to initiating a second and third bDMARD/tsDMARD were 345 days (IQR 160-835) and 770 days (IQR 395-1435), respectively.
1,745 patients were followed-up for at least 180 days following start of the initial bDMARD/tsDMARD therapy, or switched to a second bDMARD/tsDMARD within 180 days. Among these patients, 193 (11%) started a second bDMARD/tsDMARD within 180 days of the initial therapy (i.e. early switchers). 88% of these patients received TNFi as the initial therapy and 76% switched to another TNFi. Children older than 12 years were more likely to be early switchers, compared with younger children (OR 1.05; 95% CI 1.02-1.08; p< 0.001).
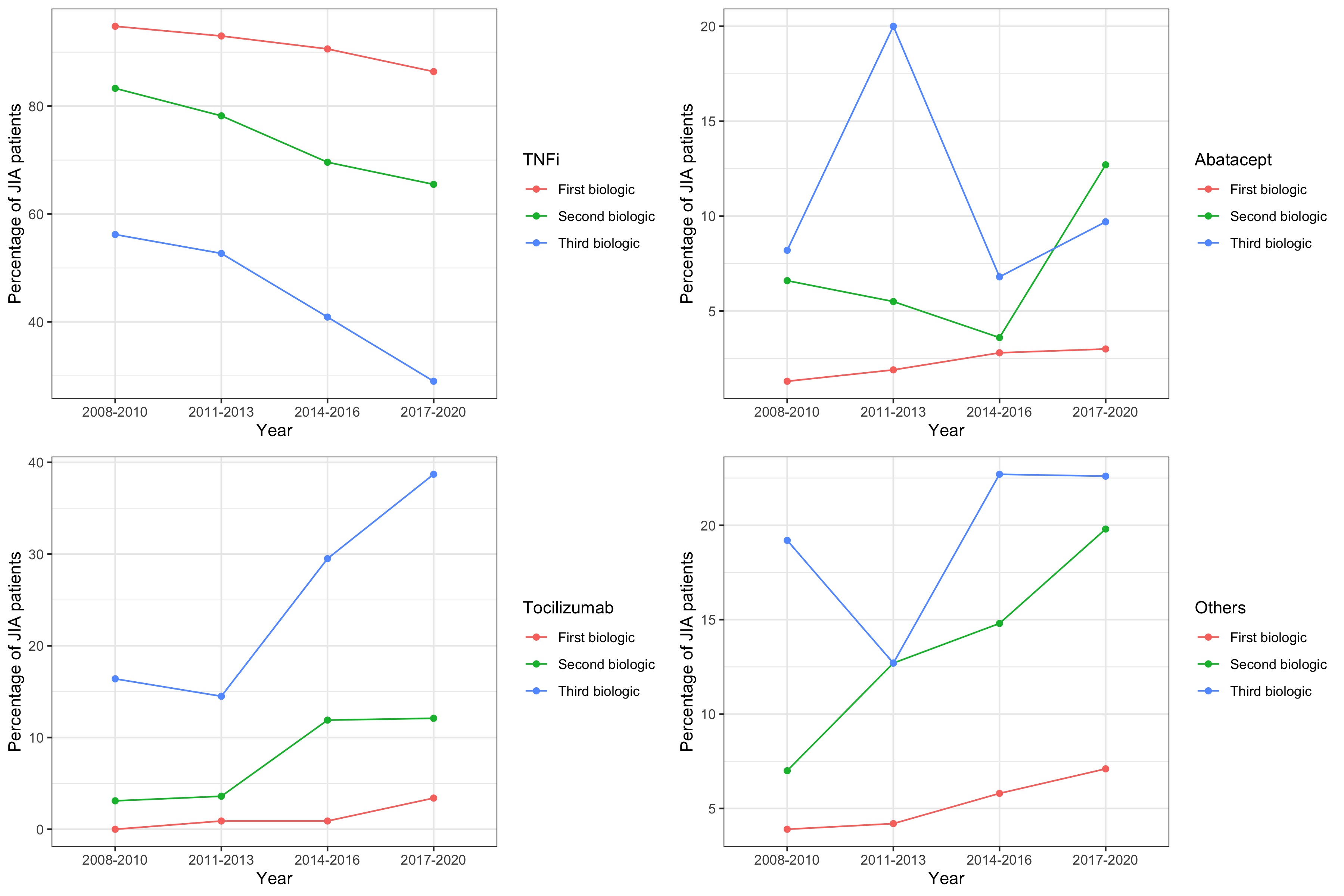
The proportion of early switchers did not vary significantly over the study period. However, changes in the types of bDMARD/tsDMARD used were observed. Notably, there was a significant reduction in the use of TNFi as initial (from 95% in 2008-2010 to 86% in 2017-2020; p< 0.001), second (from 83% in 2008-2010 to 66% in 2017-2020; p< 0.001), and third therapy (from 56% in 2008-2010 to 29% in 2017-2020; p=0.010). There was also a significant trend towards increased use of tocilizumab and other bDMARD/tsDMARD as initial and subsequent therapies (Fig 1).
Conclusion: For many children with JIA, sequential use of bDMARD/tsDMARD is common. One-third of patients in our cohort received a second therapy and one in ten patients switched within 6 months of the initial therapy. More research is needed to evaluate the outcomes and optimal sequence of therapies in this population.
To cite this abstract in AMA style:
Ong M, Ringold S, Mannion M, Natter M, Schanberg L, Kimura Y. Patterns of Medication Switching in Juvenile Idiopathic Arthritis: A Retrospective Analysis of a National Administrative Claims Database [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/patterns-of-medication-switching-in-juvenile-idiopathic-arthritis-a-retrospective-analysis-of-a-national-administrative-claims-database/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patterns-of-medication-switching-in-juvenile-idiopathic-arthritis-a-retrospective-analysis-of-a-national-administrative-claims-database/