Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Biosimilars (BioS) are cost-effective alternatives to reference products for patients with chronic inflammatory rheumatic diseases (CIRD) and inflammatory bowel diseases (IBD), but treatment adherence and maintenance after such a transition is linked to patient overall experience that could be impacted by patient or treatment characteristics. This study aimed to explore patient experience and satisfaction after the transition to a high-concentration citrate-free adalimumab biosimilar, CT-P17.
Methods: YU-MATTER (NCT05427942), a prospective observational French study included patients with chronic inflammatory rheumatic disease (CIR) or inflammatory bowel disease already treated with adalimumab (ADA), either the RP (HC: 100mg/mL) or a BioS with low concentration (LC: 50 mg/mL). Patients were switched to CT-P17 and followed-up for 3 months (M). Patient characteristics were collected at M0, as well as their treatment experience at M0, M1, M2, and M3 via 5 self-reported questionnaires developed in collaboration with patient associations to explore satisfaction regarding the injection (Likert 7), discomfort (pain [Numeric Rating Scale, NRS 0-10], redness [Likert 4], itching [NRS 0-10], and haematoma [Likert 3]). Satisfaction and overall injection tolerance (pain and itching scores < 4 + no redness + no haematoma) were assessed between M0 (just before switch) and M3 (3 months after the switch). The Last Observation Carried Forward (LOCF) method was used to replace missing data at M3. Factors associated with satisfaction improvement between M0 and M3 (increase of at least two points on the Likert 7) were searched in patients evaluable at both time-points using multivariable logistic regression models.
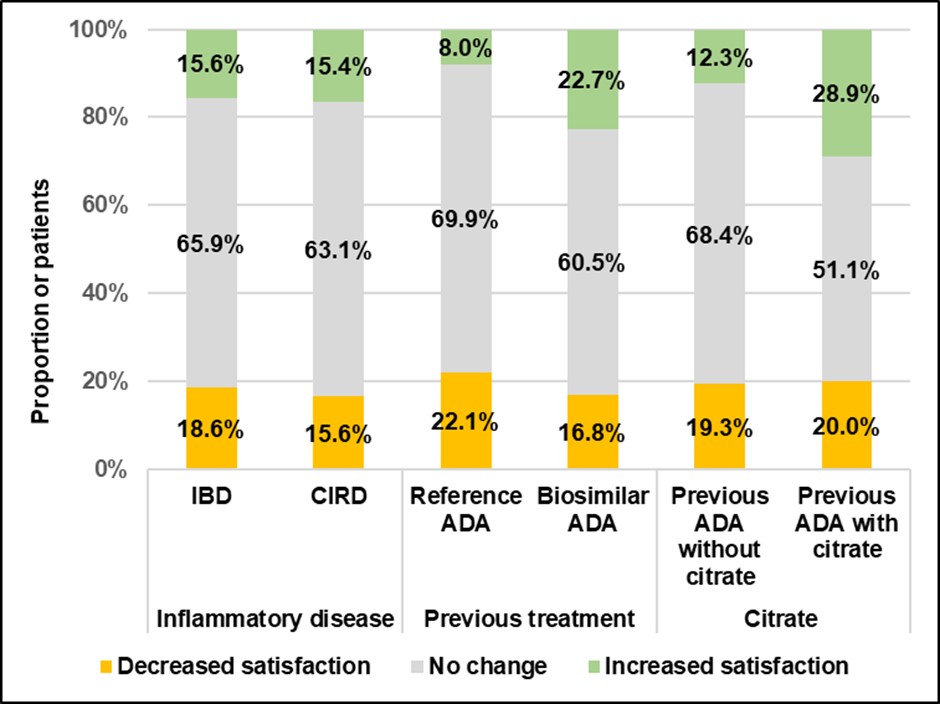
Results: 232 patients were included (CIRD: 28.0% in whom 17 rheumatoid arthritis, 35 ankylosing spondylitis, 7 non-radiographic axial spondyloarthritis and 6 psoriatic arthritis; IBD: 72.0%, in whom 136 Crohn’s disease) by 11 rheumatologists and 17 gastroenterologists. In the global population, patient age was 44±15 years, 50.4% were men, and median disease duration was 9 years (IQR: 5-16). 119 (51.2%) patients were switched from an ADA-BioS (45 with citrate, 19.4%) and 113 (48.7%) from RP-ADA. At M3, patient satisfaction did not decrease after switching. More patients reported an increased satisfaction after switching from ADA-BioS (22.7% versus 8.0%, p=0.002) or ADA with citrate (28.9% versus 12.3% p=0.008) (Figure1). Independent prognostic factors of an increased satisfaction were a previous ADA-BioS (OR=2.88; 95%CI [1.17-7.08]; p=0.021), and pain at the injection site under the previous ADA (OR=1.26 [1.08-1.47], p=0.004). Patients significantly reported less pain, redness, itching, and hematoma at M3 (p< 0.001).
Conclusion: This real-world prospective study showed that the global experience of patients after the switch to CT-P17, a high concentration and citrate-free biosimilar of the reference adalimumab, was positive with no decrease in satisfaction with treatment, and a significant improvement in overall injection tolerance.
To cite this abstract in AMA style:
Marotte H, Gossec L, ABITBOL V, SENBEL E, BONNAUD G, ROBLIN X, BOUHNIK Y, NANCEY S, MATHIEU N, FILIPPI j, VUITTON L, NAHON S, Dellal A, DENIS A, HABAUZIT C, BENKHALIFA S, BOUGUEN G. Patient Satisfaction and Experience After a Switch to a Citrate-free High-concentration Adalimumab Biosimilar. Results from a Prospective Multicentric Real-world Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/patient-satisfaction-and-experience-after-a-switch-to-a-citrate-free-high-concentration-adalimumab-biosimilar-results-from-a-prospective-multicentric-real-world-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-satisfaction-and-experience-after-a-switch-to-a-citrate-free-high-concentration-adalimumab-biosimilar-results-from-a-prospective-multicentric-real-world-study/